Sulfur hexafluoride
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Sulfur hexafluoride | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | SF 6 | |||||||||||||||||||||
| Brief description |
colorless and odorless gas |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 146.05 g mol −1 | |||||||||||||||||||||
| Physical state |
gaseous |
|||||||||||||||||||||
| density |
6.63 kg m −3 (0 ° C, 1013 hPa) |
|||||||||||||||||||||
| Sublimation point |
−63.8 ° C |
|||||||||||||||||||||
| Vapor pressure |
|
|||||||||||||||||||||
| solubility |
very bad in water (40 mg l −1 at 20 ° C, 1 bar) |
|||||||||||||||||||||
| Dipole moment |
0 |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| MAK |
Switzerland: 1000 ml m −3 or 6000 mg m −3 |
|||||||||||||||||||||
| Global warming potential |
26 087 (based on 100 years) |
|||||||||||||||||||||
| Thermodynamic properties | ||||||||||||||||||||||
| ΔH f 0 |
−1220 kJ mol −1 |
|||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Sulfur hexafluoride is an inorganic chemical compound made up of the elements sulfur and fluorine with the empirical formula SF 6 . Under normal conditions, it is a colorless, odorless, non-toxic and non-flammable gas that is extremely inert, similar to nitrogen . At normal pressure and a temperature of −63.8 ° C, sulfur hexafluoride changes directly from the solid to the gaseous state by sublimation .
Extraction and presentation
Sulfur hexafluoride can be synthesized directly from the elements by converting elemental sulfur (S 8 ) in the fluorine gas stream (F 2 ). The reaction is strongly exothermic .
In addition to SF 6 , other sulfur fluorides such as disulphur decafluoride (S 2 F 10 ) are also formed in this synthesis route . For this reason, the gas is heated to 400 ° C during technical production, which results in a disproportionation of disulfur decafluoride into sulfur hexafluoride and sulfur tetrafluoride (SF 4 ).
The sulfur tetrafluoride is destroyed by washing the gas mixture in lye , while SF 6 is not attacked by the lye.
The pure SF 6 is separated off by subsequent pressure distillation.
properties
Physical Properties
Sulfur hexafluoride is gaseous under normal conditions. It is about five times as dense as air . Its sublimation point is −63.8 ° C.
The triple point is at a temperature of −50.8 ° C and a pressure of 2.26 bar. A liquid phase is only possible above this pressure.
The critical point lies at a temperature of 45.6 ° C, a critical pressure of 3.76 MPa and a critical density of 0.735 g · cm −3 .
The enthalpy of vaporization of sulfur hexafluoride is 115 kJ / kg at a pressure of 1013 hPa.
Crystal and molecular structure
At low temperatures it crystallizes in the monoclinic crystal system .
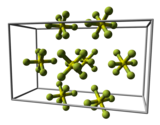
In the gaseous state, the SF 6 molecule is octahedral ( O h ); the S – F bond length is 156.1 pm .
Chemical properties
Due to its structure, it is practically chemically inert and therefore behaves in a similar way to molecular nitrogen or noble gases . It is almost insoluble in water and non-flammable.
Due to its inertness, reactions can usually only be carried out under more drastic than normal conditions. So sit alkali metals with SF 6 in liquid ammonia to the corresponding sulfides and fluorides to:
- .
In the presence of hydrogen sulfide , the comproportionation to elemental sulfur and hydrogen fluoride (HF) is known:
- .
SF 6 is isoelectronic to the anions hexafluorophosphate (PF 6 - ), hexafluorosilicate (SiF 6 2− ) and hexafluoroaluminate (AlF 6 3− ).
use

Sulfur hexafluoride (SF 6 ) is used as an insulating gas in medium and high voltage technology, for example in gas-insulated switchgear (GIS) with high- voltage switches and in gas-insulated pipelines (GIL) in completely encapsulated systems with operating voltages from 6 kV to 800 kV. Compared to outdoor switchgear, space is saved significantly and the effects of weather and birds or rodents are avoided. SF 6 also serves as an extinguishing gas to interrupt the switching arc in circuit breakers .
The dielectric strength at normal pressure is almost three times as high as in air or nitrogen. These properties and low dielectric losses make it ideal for use as an insulating gas in coaxial cables and gas-insulated high-frequency - power capacitors , which can thus be made smaller. As an insulating gas in electrical switchgear, it is kept under increased pressure of 5 bar to 10 bar in order to ensure the necessary high insulation capacity. The increased gas pressure is necessary because it reduces the mean path length of the free electrons in the gas according to Paschen's law . This means that electrons cannot be accelerated as strongly as at normal pressure, because they collide with the SF 6 molecules earlier .
In switchgear with the basically non-toxic SF 6 gas, the arcs in combination with impurities such as a low water content, in addition to the non-toxic tetrafluoromethane, result in toxic fluoride compounds such as hydrogen fluoride and thionyl fluoride as well as the highly toxic disulfur decafluoride (S 2 F 10 ). For these reasons, appropriate safety guidelines for venting must be observed in gas-tight SF 6 switchgear before maintenance work.
It is used as an insulating gas for routine tests (checking) of microelectronic circuits as part of quality assurance.
In the manufacture of semiconductor components, it is used as an etching gas: SF 6 is the reactive gas in reactive ion etching ( RIE ) and DRIE ( deep reactive ion etching ). It is also used in a similar way for cleaning etching, for example in display production.
SF 6 is also used as a protective gas in the production of magnesium . The SF 6 , specifically heavier than air, prevents the hot metal melt from coming into contact with the ambient air. Due to the process, very large amounts of SF 6 are released into the atmosphere in this application .
SF 6 was previously used as an insulating gas between insulating glass panes and as a filling gas in the soles of sports shoes. In addition, until around the year 2000, sulfur hexafluoride was also used to fill car tires, although this cost up to DM 100 per set of tires (approx. 50 euros ) (see also tire gas ). All three of the above applications are now prohibited for reasons of environmental protection.
Due to its physical properties, the low background concentration in the atmosphere and the very good traceability in gas analyzers SF 6 currently still as a tracer gas for ventilation efficiency - measurements used in very small quantities. For many applications, however, fewer climate-damaging gases are now used.
In the ophthalmology a is a mixture of sulfur hexafluoride and air against retinal detachment employed to a reapplication of the retina to reach. For this purpose, during the surgical removal of the vitreous body ( vitrectomy ), the gas mixture is introduced into the vitreous cavity ( camera vitrea bulbi ) for the purpose of pressing the retina onto its surface (endotamponade).
SF 6 has been used as an ultrasound contrast agent in medicine since 2001 . Here it is used in particular to detect liver metastases from malignant tumors. The advantages are a very high temporal and spatial contrast resolution. Thyroid diseases and kidney insufficiency are not a contraindication for carrying out this examination. With the help of SF 6 , liver foci can be correctly detected to about 90%.
Climate relevance

SF 6 gas is loud 's Fifth Assessment Report of the IPCC ( Intergovernmental Panel on Climate Change , Intergovernmental Panel on Climate Change ), the strongest known greenhouse gas . Over a period of 100 years, 1 kg of this gas is just as effective as 26 087 kg of carbon dioxide (CO 2 ). The atmospheric lifespan of SF 6 is approximately 3,200 years.
Because of the very low concentration of SF 6 in the earth's atmosphere (approx. 10 ppt ( parts per trillion ) in terms of volume in 2019, which corresponds to 0.78 ppmV CO 2 equivalent; approx. 0.3 ppt increase per year; CO 2 approx. 400 ppm with an increase of approx. 2 ppm per year per year), its influence on global warming is still considered to be relatively moderate. It does not contribute to the destruction of the ozone layer.
In 1997, emissions from electrical engineering systems in Germany amounted to 10% of 238 t of total emissions. The relative share of the electrical industry in emissions fluctuates strongly between different countries and, according to data reported by the industry, varies between 20–30% (EU) and 70–80% (US) in the period 1990–2012.
The increase in the SF 6 concentration in the last few years at the Bukit Kototabang station in Indonesia has been from 5.3 ppt at the beginning of 2004 to 6.3 ppt at the end of 2008, which corresponds to an increase of around 19% in five years.
In 2016, 1142 tonnes of sulfur hexafluoride were sold to users in Germany, which is around 2% or 23 t more than in 2015. The climate impact of the amount in 2016 is 26 million t of CO 2 equivalents , with some of this amount also in closed systems were filled. For this calculation, the greenhouse effect factor 22,800 compared to CO 2 was used for a time horizon of 100 years. The main amount went to the electrical industry and apparatus construction with 21.9 million tons of CO 2 equivalents for 2016, followed by the semiconductor industry with 1.2 million tons of CO 2 equivalents.
Emissions
| Emission source | 1990 | 1995 | 2000 | 2002 | 2004 | 2006 | 2007 | 2007 potential emissions * |
|---|---|---|---|---|---|---|---|---|
| Cast aluminum / trace gas | 1 | 1 | 14.5 | 35.5 | 46 | 85.5 | 84 | - |
| Soundproof windows | 69 | 108 | 52 | 46 | 54 | 61 | 67 | 1950 |
| Solar technology / opt. Fibers | 0 | 0 | 0 | 0.4 | 1.5 | 4.7 | 20.3 | - |
| Electrical switchgear | 23 | 27.3 | 16.9 | 15.7 | 16.3 | 14.4 | 15.8 | 1770 |
| Magnesium foundries | 7.4 | 7.7 | 13.4 | 16.1 | 24.9 | 24.1 | 15.2 | - |
| T&D components | k. A. | 16.7 | 26.6 | 23.3 | 16.0 | 12.4 | 9.9 | k. A. |
| Particle accelerator | 5.2 | 4.5 | 5.0 | 4.9 | 4.9 | 4.9 | 4.9 | 74 |
| car tire | 65 | 110 | 50 | 9 | 4th | 2.5 | 2 | 6th |
| Semiconductor production | 3.7 | 2 | 2.4 | 2.4 | 3.4 | 1.3 | 1.2 | - |
| Others | 11 | 26th | 32 | 24 | 21st | 20th | 13 | k. A. |
| All in all | 200 | 300 | 210 | 180 | 190 | 230 | 230 | - |
| * average annual stock | ||||||||
Oddities
Because of its approximately five times higher density than normal air, sulfur hexafluoride can be poured into containers like an invisible liquid. Very light objects, such as bowls made of aluminum foil, can then “swim” on the SF 6 mirror. Such an experiment succeeds with great care even with more easily accessible CO 2 .
Inhaled SF 6 gives a voice that is lower than air when speaking - in contrast to helium , for example . The reason for this is the significantly higher density of SF 6 compared to air , which leads to a lower speed of sound in the gas (129 m / s, factor 0.39 compared to air).
Experiments with it are risky, however, since SF 6 in the lungs hinders the exhalation of carbon dioxide. The risk of carbon dioxide anesthesia or respiratory arrest is therefore greater with SF 6 than, for example, with nitrogen or helium.
Occasionally it is claimed that because of its high density , SF 6 can only be exhaled while standing upside down. This is not correct, because the gases in the lungs mix as a result of the breathing currents, so the SF 6 can be exhaled normally.
literature
- AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , pp. 566-567.
Web links
- International Chemical Safety Card (ICSC) for SULPHUR HEXAFLUORIDE at the National Institute for Occupational Safety and Health (NIOSH).
- Video of the two effects in the Curiosities section
Individual evidence
- ↑ a b c d e f g h i j k Entry on sulfur hexafluoride in the GESTIS substance database of the IFA , accessed on July 8, 2019(JavaScript required) .
- ↑ Safety data sheet (praxair) ( Memento from September 27, 2007 in the Internet Archive ) (PDF; 36 kB).
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Permittivity (Dielectric Constant) of Gases, pp. 6-188.
- ↑ Schweizerische Unfallversicherungsanstalt (Suva): Limits - Current MAK and BAT values (search for 2551-62-4 or sulfur hexafluoride ), accessed on November 2, 2015.
- ↑ a b G. Myhre, D. Shindell et al .: Climate Change 2013: The Physical Science Basis . Working Group I contribution to the IPCC Fifth Assessment Report. Ed .: Intergovernmental Panel on Climate Change . 2013, Chapter 8: Anthropogenic and Natural Radiative Forcing, pp. 24-39; Table 8.SM.16 ( PDF ).
- ↑ a b Chase, MW, Jr., NIST-JANAF Themochemical Tables, Fourth Edition, J. Phys. Chem. Ref. Data , Monograph 9, 1998, 1-1951.
- ↑ Walter C. Schumb: Sulfur (VI) fluoride . In: Ludwig F. Audrieth (Ed.): Inorganic Syntheses . tape 3 . McGraw-Hill, Inc., 1950, pp. 119-124 (English).
- ↑ A. Lieberam: Caloric and critical data. In: Association of German Engineers, VDI Society for Process Engineering and Chemical Engineering (ed.): VDI-Wärmeatlas. Calculation sheets for heat transfer. 7th expanded edition. VDI-Verlag, Düsseldorf 1994, ISBN 3-18-401362-6 , S. Dc1.
- ^ MT Dove, BM Powell, GS Pawley, LS Bartell: Monoclinic phase of SF 6 and the orientational ordering transition. In: Molecular Physics , 1988, 65 (2), pp. 353-358 ( doi : 10.1080 / 00268978800101081 ).
- ↑ LS Bartell, SK Doun: Structures of hexacoordinate compounds of main-group elements. Part III. An electron diffraction study of SF 6 , in: Journal of Molecular Structure , 1978, 43 , pp. 245-249 ( doi : 10.1016 / 0022-2860 (78) 80010-6 ).
- ↑ Holger Deubner, Florian Kraus, Holger Lars Deubner, Florian Kraus: The Decomposition Products of Sulfur Hexafluoride (SF6) with Metals Dissolved in Liquid Ammonia . In: Inorganics . tape 5 , no. 4 , October 13, 2017, p. 68 , doi : 10.3390 / inorganics5040068 ( mdpi.com [accessed September 18, 2018]).
- ^ HM Ryan: SF 6 switchgear . IET, 1989, ISBN 978-0-86341-123-6 , pp. 122 ff . ( limited preview in Google Book search).
- ↑ H. Rebholz, W. Köhler, S. Tenbohlen: Dielectric strength of different gases in GIS . University of Stuttgart, 2005 ( PDF ; 396 kB).
- ↑ SF 6 systems (PDF; 356 kB), Professional Association for Precision Mechanics and Electrical Engineering, May 2008.
- ↑ V. Boudon, J.-P. Champion, T. Gabard, G. Pierre, M. Loëte, C. Wenger: Spectroscopic tools for remote sensing of greenhouse gases CH 4 , CF 4 and SF 6 . In: Environmental Chemistry Letters , March 2003, Volume 1, Issue 1, pp. 86–91 ( online as PDF, 300 KiB ).
- ↑ Danger from the sneaker. In: Greenpeace Magazin , 2.98.
- ↑ Chemical Risk Reduction Ordinance, Appendix 1.5 .
- ↑ Regulation (EC) No. 842/2006 on certain fluorinated greenhouse gases , Art. 8 f. and Appendix II (PDF) .
- ↑ Galle, B .; Samuelsson, J .; Svensson, BH and Borjesson G: Measurements of Methane Emissions from Landfills Using a Time Correlated Tracer Method Based on FTIR Absorption Spectroscopy. In: Environmental Science & Technology , 2001, Vol. 35, No. 1, pp. 21-25; doi : 10.1021 / es0011008 .
- ^ Franz Grehn: Ophthalmology . Springer-Verlag, Heidelberg 2006, ISBN 978-3-540-25699-1 , p. 190–211 ( limited preview in Google Book search).
- ^ D. Strobel, K. Seitz, W. Blank, A. Schuler, C. Dietrich, A. von Herbay, M. Friedrich-Rust, G. Kunze, D. Becker, U. Will, W. Kratzer, FW Albert , C. Pachmann, K. Dirks, H. Strunk, C. Greis, T. Bernatik: Contrast-enhanced ultrasound for the characterization of focal liver lesions - diagnostic accuracy in clinical practice (DEGUM multicenter trial). In: Ultrasound in Medicine. Volume 29, Number 5, October 2008, pp. 499-505, doi : 10.1055 / s-2008-1027806 , PMID 19241506 .
- ↑ PEMANTAUAN GAS RUMAH KACA ( Memento from April 24, 2012 in the Internet Archive ).
- ↑ Destatis: Sales of sulfur hexafluoride increased in 2016 . In: UmweltMagazin . 47, No. 6, 2017, ISSN 0173-363X , p. 12.
- ↑ Umweltbundesamt (Ed.): Avoiding fluorinated greenhouse gases - ways to get out . August 2010, p. 37 - Table 2.5 ( Umweltbundesamt.de - German version ).
- ↑ Federal Government (Ed.): Report by the Federal Republic of Germany in accordance with Articles 5, 7 and 8 of the Kyoto Protocol of the UN Framework Convention on Climate Change on fluorinated greenhouse gases to the Secretariat of the Framework Convention on Climate Change in 2009. April 2009 ( unfccc.int ).
- ^ Winfried Schwarz: Emissions of fluorinated greenhouse gases in Germany 2006 and 2007 - inventory determination 2006/2007 (F-gases). Data from HFC, PFC and SF6 for national reporting in accordance with the Framework Convention on Climate Change for the reporting years 2006 and 2007 as well as verification of data collection via external databases. Ed .: Federal Environment Agency. Texts No. 22/2009, 2009 ( Umweltbundesamt.de ).