Topicity: Difference between revisions
"is" |
m de: |
||
| Line 32: | Line 32: | ||
[[Category:Stereochemistry]] |
[[Category:Stereochemistry]] |
||
[[de:Topizität]] |
|||
Revision as of 16:22, 27 September 2007
In chemistry, topicity is the stereochemical relationship of substituents relative to the structure to which they are attached. Depending on the relationship, such groups can be homotopic, enantiotopic, or diastereotopic.
Homotopic
Homotopic groups in a chemical compound are equivalent groups. Two groups A and B are homotopic if the molecule remains the same (including stereochemically) when the groups are interchanged with the remaining parts of the molecule fixed. Homotopic atoms have the same chemical shift in an NMR spectrum.
Enantiotopic
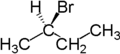
The stereochemical term enantiotopic refers to the relationship between two groups attached to the same atom which, if replaced, would generate compounds that are enantiomers.
For example, the two hydrogen atoms attached to the second carbon in butane are enantiotopic. Replacement of one hydrogen atom (colored blue) with a bromine atom will produce (R)-2-bromobutane. Replacement of the other hydrogen atom (colored red) with a bromine atom will produce the enantiomer (S)-2-bromobutane.
 |
 |

|
| Butane | (R)-2-bromobutane | (S)-2-bromobutane |
Diastereotopic
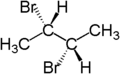
The stereochemical term diastereotopic refers to the relationship between two groups attached to the same atom which, if replaced, would generate compounds that are diastereomers.
For example, the two hydrogen atoms attached to the third carbon in (S)-2-bromobutane are diastereotopic. Replacement of one hydrogen atom (colored blue) with a bromine atom will produce (2S,3R)-2,3-dibromobutane. Replacement of the other hydrogen atom (colored red) with a bromine atom will produce the diastereomer (2S,3S)-2,3-dibromobutane.
 |
 |
|
| (S)-2-bromobutane | (2S,3R)-2,3-dibromobutane | (2S,3S)-2,3-dibromobutane |
