Sulfonamide

In chemistry, the sulfonamide functional group is -S(=O)2-NH2, a sulfone group connected to an amine group.
A sulfonamide (compound) is a compound that contains this group. The general formula is RSO2NH2, where R is some organic group. For example, "methanesulfonamide" is CH3SO2NH2. Any sulfonamide can be considered as derived from a sulfonic acid by replacing a hydroxyl group with an amine group.
In medicine, the term "sulfonamide" is sometimes used as a synonym for sulfa drug, a derivative or variation of sulfanilamide.
Organic synthesis
Sulfonamides can be prepared in the laboratory in many ways:
- by reaction of sulfonyl chlorides with amines for example in the synthesis of sulfonylmethylamide [1]. A readily available sulfonyl chloride source is tosyl chloride [2]
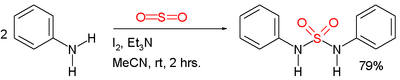
- In one study symmetric sulfonamides are prepared directly from amines and sulfur dioxide gas [3]:
In this example the reactant is aniline and the catalysts are triethylamine and iodine. Sulfur dioxide is believed to be activated through a series of intermediates: Et3N-I+-I-, Et3N-I+-I3- and Et3N+-SO2-.
See also
References
- ^ Organic Syntheses, Coll. Vol. 4, p.943 (1963); Vol. 34, p.96 (1954). Online Article
- ^ Organic Syntheses, Coll. Vol. 5, p.39 (1973); Vol. 48, p.8 (1968). Online Article
- ^ Sulfamides and sulfamide polymers directly from sulfur dioxide Alexander V. Leontiev, H. V. Rasika Dias and Dmitry M. Rudkevich Chem. Commun., 2006, 2887 - 2889, doi:10.1039/b605063h