Immunoglobulin heavy chain: Difference between revisions
changed .png image to .svg |
|||
| Line 9: | Line 9: | ||
===Regions=== |
===Regions=== |
||
[[Image:AntibodyChains.svg|thumb|Schematic diagram of |
[[Image:AntibodyChains.svg|thumb|Schematic diagram of a typical antibody showing two Ig heavy chains (blue) linked by [[disulphide bond]]s to two Ig light chains (green). The constant (C) and variable (V) domains are shown.]] |
||
Each heavy chain has two regions: |
Each heavy chain has two regions: |
||
* a ''constant region'' (which is the same for all immunoglobulins of the same class but differs between each class of immunoglobulins). |
* a ''constant region'' (which is the same for all immunoglobulins of the same class but differs between each class of immunoglobulins). |
||
Revision as of 00:35, 18 September 2007
A heavy chain is the large polypeptide subunit of an antibody (or immunoglobulin); a typical antibody is composed of two immunoglobulin (Ig) heavy chains and two Ig light chains. Several different types of heavy chain exist that define the class or isotype of an antibody. These heavy chain types vary between different animals. All heavy chains contain a series of immunoglobulin domains, usually with one variable (IgV) domain that is important for binding antigen and several constant (IgC) domains.
In mammals
Classes
There are five types of mammalian immunoglobulin heavy chain: γ, δ, α, μ and ε.[1] They define classes of immunoglobulins.
- Heavy chains α and γ have approximately 450 amino acids.
- Heavy chains μ and ε have approximately 550 amino acids. [1]
Regions
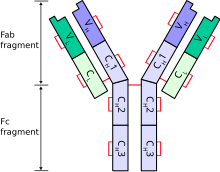
Each heavy chain has two regions:
- a constant region (which is the same for all immunoglobulins of the same class but differs between each class of immunoglobulins).
- a variable region that differs between different B cells, but is the same for all immunoglobulins produced by the same B cell or B cell clone. The variable domain of any heavy chain is composed of a single immunoglobulin domain. These domains are about 110 amino acids long.
In fish
Jawed fish appear to be the most primitive animals that are able to make antibodies like those described for mammals.[3] However, fish do not have the same repertoire of antibodies that mammals possess.[4] Three distinct Ig heavy chains have so far been identified in bony fish.
- The first identified was the μ (or mu) heavy chain that is present in all jawed fish and is the heavy chain for what is thought to be the primordial immunoglobulin. The resulting antibody, IgM, is secreted as a tetramer (containing four polypeptide chains) in teleost fish instead of the typical pentamer (containing five polypeptide chains) found in mammals and sharks.
- The heavy chain (δ) for IgD was identified initially from the channel catfish and Atlantic salmon and is now well documented for many teleost fish.[5]
- The third teleost Ig heavy chain gene was identified very recently and does not resemble any of the heavy chains so far described for mammals. This heavy chain, identified in both rainbow trout (τ)[6] and zebrafish (ζ),[7] could potentially form a distinct antibody isotype (IgT or IgZ) that may precede IgM in evolutionary terms.
Similar to the situation observed for bony fish, three distinct Ig heavy chain isotypes have been identified in cartilaginous fish. With the exception of IgM, these Ig heavy chain isotypes appear to be unique to cartilaginous fish and are designated IgM, IgW (also called IgX or IgNARC) and IgNAR.[8]
References
- ^ a b c Janeway CA, Jr.; et al. (2001). Immunobiology (5th ed. ed.). Garland Publishing. (electronic full text via NCBI Bookshelf) ISBN 0-8153-3642-X.
{{cite book}}:|edition=has extra text (help); Explicit use of et al. in:|author=(help) - ^ Woof J, Burton D (2004). "Human antibody-Fc receptor interactions illuminated by crystal structures". Nat Rev Immunol. 4 (2): 89–99. PMID 15040582.
- ^ Fish heavy chain and light chain genes
- ^ Eva Bengtén, L. William Clem, Norman W. Miller, Gregory W. Warr and Melanie Wilson. Channel catfish immunoglobulins: Repertoire and expression. Developmental & Comparative Immunology, Volume 30, Issues 1-2, Antibody repertoire development, 2006, Pages 77-92.
- ^ Stein Tore Solem and Jørgen Stenvik. Antibody repertoire development in teleosts--a review with emphasis on salmonids and Gadus morhua L. Developmental & Comparative Immunology, Volume 30, Issues 1-2, Antibody repertoire development, 2006, Pages 57-76.
- ^ J.D. Hansen, E.D. Landis and R.B. Phillips. Discovery of a unique Ig heavy-chain isotype (IgT) in rainbow trout: Implications for a distinctive B cell developmental pathway in teleost fish. Proceedings of the National Academy of Sciences U S A. Volume 102, Issue 19, 2005, pages 6919-24.
- ^ N. Danilova, J. Bussmann, K. Jekosch, L.A Steiner. The immunoglobulin heavy-chain locus in zebrafish: identification and expression of a previously unknown isotype, immunoglobulin Z. Nature Immunology, Volume 6, Issue 3, 2005, pages 295-302.
- ^ H. Dooley and M.F. Flajnik. Antibody repertoire development in cartilaginous fish. Developmental & Comparative Immunology, Volume 30, Issues 1-2, Antibody repertoire development, 2006, Pages 43-56.
External links
- Immunoglobulin+Heavy+Chains at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
