Lucigenin

| |
| Names | |
|---|---|
| Preferred IUPAC name
10,10′-Dimethyl[9,9′-biacridine]-10,10′-diium dinitrate | |
| Other names
Bis-N-methylacridinium nitrate; N,N′-Dimethyl-9,9'-bisacridinium nitrate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.017.295 |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C28H22N4O6 | |
| Molar mass | 510.506 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Lucigenin is an aromatic compound used in areas which include chemiluminescence. Its chemical name is bis-N-methylacridinium nitrate. It exhibits a bluish-green fluorescence.
It is used as a probe for superoxide anion in biology, for its chemiluminescent properties.
Synthesis
It may be prepared from acridone.
There's also a route from toluene:[1]
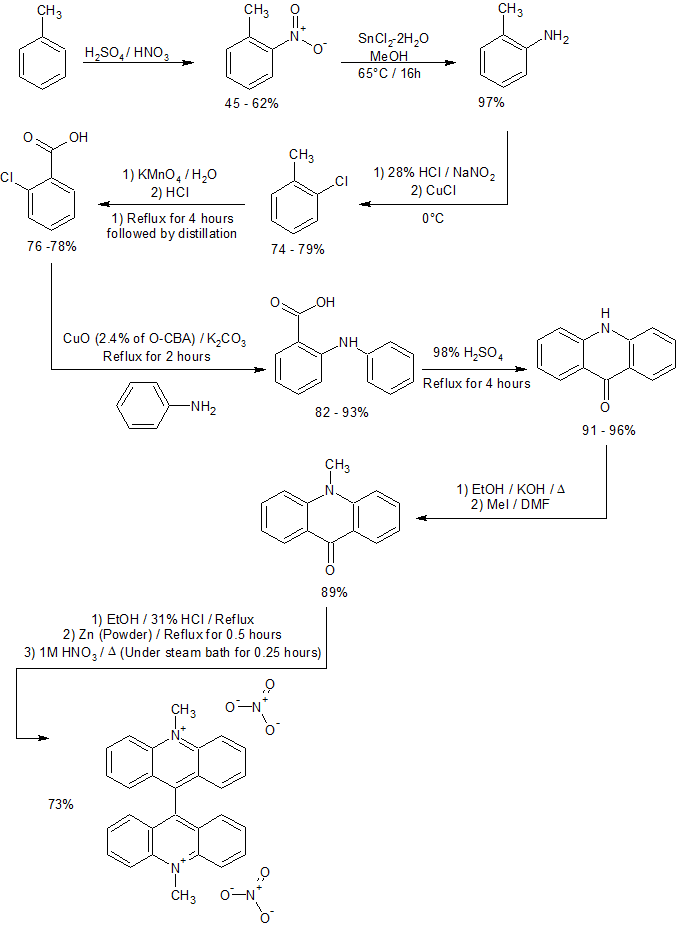
References
- ^ Amiet, R. Gary (February 1982). "The preparation of lucigenin: An experiment with charm". Journal of Chemical Education. 59 (2): 163–164. doi:10.1021/ed059p163. ISSN 0021-9584. Retrieved 20 November 2023.
