Angeli Rimini reaction
The Angeli-Rimini reaction is a specific detection reaction for aldehydes , in which the reaction of the aldehyde with a derivative of hydroxylamine produces a hydroxamic acid that forms an intensely red complex with iron (III) chloride . The reaction was discovered by the Italian chemists Angelo Angeli (1864–1931) and Enrico Rimini and published in 1896.

- The Angeli-Rimini reaction, here with benzenesulfhydroxamic acid (benzenesulfonic acid hydroxylamide, N -hydroxybenzenesulfonamide) as the reagent
mechanism
Several reaction pathways are discussed for the exact course of hydroxamic acid formation:
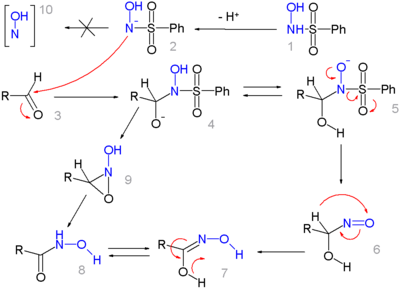
The N -hydroxybenzoesulfonamide 1 or the deprotonated form 2 acts as a nucleophile in the reaction with the aldehyde 3 to form intermediate 4 . After intramolecular proton exchange to form 5 , a benzenesulfinate anion is split off and hydroxamic acid 8 is formed via the nitrone 6 and the intermediate 7 .
Alternatively, it is discussed that after cleavage of the sulfinic acid anion, an aziridine intermediate 9 is formed from 4 , which further reacts directly to form hydroxamic acid 8 .
The occurrence of hydroxyl nitrene (NOH, 10 ), on the other hand, could definitely be ruled out.
Originally, the unstable nitrohydroxylamine or its disodium salt (disodium trioxodinitrate (II), Angeli salt ) was used as the reagent ; here the nitrite ion acts as a leaving group. Since this salt decomposes with the formation of the very unstable nitroso hydrogen (HNO, nitroxyl), a reaction mechanism is also discussed that proceeds via free nitroxyl.
Individual evidence
- ↑ Entry on Angeli-Rimini reaction. In: Römpp Online . Georg Thieme Verlag, accessed on June 14, 2014.
- ↑ Angelo Angeli, Gazz. Chim. Ital. 1896 , 26, 17.
- ↑ Rimini, E. Gazz. Chim. Ital. 1901 , 31, 84.
- ↑ Alfred Hassner, E. Wiederkehr, AJ Kascheres: Reaction of aldehydes with N-hydroxybenzenesulfonamide. Acetal formation catalyzed by nucleophiles, J. Org. Chem. 1970 ; 35 (6), pp. 1962-1964, ( doi : 10.1021 / jo00831a052 ).
- ↑ Paul Karrer, Textbook of Organic Chemistry, 7th edition, Georg Thieme Verlag 1941.
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 101st edition. Walter de Gruyter, Berlin 1995, ISBN 3-11-012641-9 .