Cornforth rearrangement
The Cornforth rearrangement is a name reaction in organic chemistry and was discovered in 1949 by the Australian chemist John W. Cornforth . This is the thermal rearrangement of carbonyl-substituted 1,3-oxazoles to isomeric 1,3-oxazoles.
Overview reaction
In the original work, John W. Cornforth used 2-phenyl-5-ethoxyoxazole-4-carboxamide (R 1 = phenyl group, R 2 = ethoxy group, R 3 = amino group ).
However, the reaction also takes place with a large number of other carbonyl-substituted 1,3-oxazoles.
Michael Dewar researched the reaction further in the early 1970s . It turned out that the reaction achieved particularly good yields of more than 90% when using nitrogen-containing heterocycles (R 3 = N-heterocycle).
mechanism
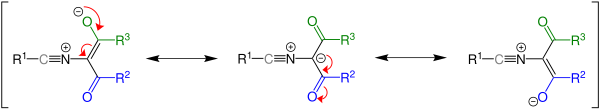
The main step of the mechanism is a pericyclic ring opening and subsequent rearrangement.
If the 1,3-oxazole used is heated, the dicarbonyl nitrile 1 is formed through ring opening . This intermediate cannot be isolated. The oxygen atom then attacks the carbon atom of the nitrile group and closes the ring again.
Whether the reaction takes place at all depends on the energy difference between the starting material and the product.
The substituents R 2 and R 3 are decisive for this , as they influence the isomerization of the zwitterion shown.
application
The Cornforth rearrangement finds application in the synthesis of amino acids. Here the corresponding oxazoles occur as intermediates.
Individual evidence
- ↑ a b J.W. Cornforth, E. Fawaz, LJ Goldsworthy, Robert Robinson: 330. A synthesis of acylamidomalondialdehydes . In: Journal of the Chemical Society (Resumed) . 1949, p. 1549-1553 , doi : 10.1039 / JR9490001549 .
- ↑ a b c d e László Kürti, Barbara Czakó: Strategic Applications of Named Reactions in Organic Synthesis: Background and Detailed Mechanisms . Elsevier Academic Press, 2005, ISBN 978-0-12-429785-2 , pp. 112-113.
- ↑ Michael JS Dewar: Cornforth rearrangement . In: Journal of the American Chemical Society . tape 96 , no. September 19 , 1974, p. 6148-6152 , doi : 10.1021 / ja00826a030 .
- ^ Jie Jack Li: Name Reactions - A Collection of Detailed Reaction Mechanisms . Springer, 2006, ISBN 978-3-540-30030-4 , p. 95.