Curtin-Hammett principle
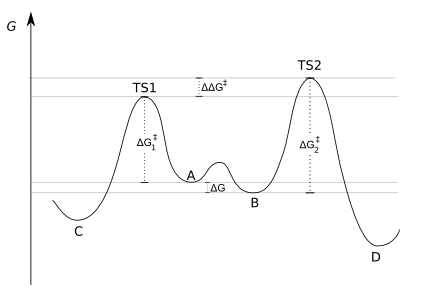
The Curtin-Hammett principle is a principle in chemical kinetics established by David Yarrow Curtin and Louis Plack Hammett . It says that in a chemical reaction, the position of an upstream rapid equilibrium does not influence product formation. If two reactive intermediates A and B are in equilibrium with each other (for example conformers) and one of these intermediates (A) can convert into a product C or the other (B) into D, the product ratio depends only on the free energy of the transition state respective product, but not on the equilibrium constant K between A and B. A and B are in dynamic equilibrium, A is irreversibly converted into C, B irreversibly into D.
K is the equilibrium constant between A and B, k 1 and k 2 are the rate constants for the formation of C and D. If the reaction rate of the conversion from A to B and vice versa is greater than k 1 or k 2 , says the Curtin-Hammett - Principle that the product ratio C: D does not depend on K, but on the relative energies of the transition states.
The energy scheme can be represented as follows:
The product ratio only depends on ΔΔG ‡ (see scheme): C is the main product, because the energy of the transition state TS 1 is lower than that of the state TS 2 . It does not matter whether there is more of A or B in equilibrium.