Defect concentration
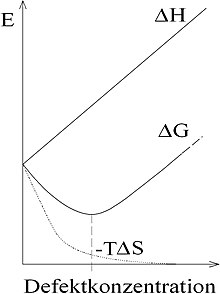
As defect concentration is the ratio of the number of defects in the lattice structure of a solid to the total number of the lattice sites. Lattice defects in a solid occur because they bring an entropy gain through disorder. For a given defect concentration c , the free enthalpy can be determined using the Gibbs-Helmholtz equation :
The incorporation of imperfections costs reaction energy and generates entropy for it . The function runs through a minimum, because for small c grows faster than . If the defects can be generated in any quantity (e.g. vacancies ) or are available (e.g. diffusion of foreign atoms from another phase ), the defect concentration will aim for the value at which the minimum assumes. Whether and when the equilibrium value is reached depends on how fast the diffusion takes place.
As the temperature rises, the entropic curve ( ) becomes steeper, which means that the minimum free energy shifts to higher defect concentrations.
Only zero-dimensional lattice errors are in thermodynamic equilibrium. Other lattice defects (e.g. dislocations and interfaces) can, however, be mechanically stable, so that they cannot be completely eliminated even by prolonged annealing.
literature
- Lesley Smart, Elaine Moore: An Introduction to Solid State Chemistry . Springer, 1997, ISBN 3-540-67066-1 , pp. 148-151 .