Dose adjustment in renal insufficiency
Dose adjustment in renal insufficiency , DANI for short , refers to the adjustment of drug dosages in patients with impaired renal function. The basis for such dose adjustments are clinical studies and / or rule-based calculations based on pharmacokinetic parameters. The reason for this is that many drugs are excreted more slowly in renal insufficiency . With a normal (not adjusted) dosage, the drug can accumulate more intensely in the body and thus safe concentrations of the drug can be exceeded, which can lead to increased side effects and even organ damage. Therefore, in patients with renal insufficiency, the drug dose and / or the frequency of use of certain drugs must be reduced.
Theoretical background
The pharmacokinetics of a medicinal substance describe, among other things, how quickly and to what extent after the administration of a medicinal substance it occurs in the blood plasma and in the various body tissues and where and in what way it is excreted ( eliminated ) again. The latter can happen , for example, via the liver and the biliary tract through the stool (hepatic / fecal ) and / or the kidneys through the urine ( renal ). The elimination capacity of the kidneys is quantified with the parameter renal clearance . In patients with renal insufficiency, the elimination of various drugs can be impaired. To what extent depends on the so-called individual elimination capacity of the patient, which can be estimated based on the extrarenal dose fraction (Qo) .
Drug clearance and extrarenal dose fraction (Qo)
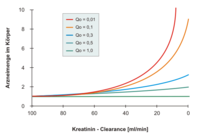
The body's ability (capacity) to excrete a drug in a given time is quantified by total drug clearance - it is the sum of the non-renal (often mostly hepatic) and renal clearances . The latter depends on the overall function of the kidneys, as can be measured, for example, using creatinine clearance. If the kidney function worsens, the elimination of the renal component usually falls in proportion to the creatinine clearance. The extrarenal dose fraction (Qo) is a key figure for the proportion of the drug that is not excreted by the kidneys. describes the share of the kidneys in the total drug clearance with normal kidney function (= 'renal elimination fraction'). The Qo value varies from substance to substance.
As a rule of thumb, the higher the Qo value of a drug, the lower its dose has to be adjusted in the case of renal insufficiency. The main restriction is that the Qo value generally relates to the parent substance, so that the Qo value for drugs with active metabolites (e.g. glibenclamide, pethidine) is not meaningful with regard to dose adjustment.
Individual Elimination Capacity (Q)
In patients with renal insufficiency, the so-called individual elimination capacity (Q) can be calculated using the Dettli formula . The patient's renal function is in this case (engl. By the estimated estimated ) Glomerular filtration rate (GFR) described below:
Calculation of the DANI
There are several ways to calculate a dose adjustment. With knowledge of the individual elimination capacity (Q) with regard to a certain medicinal substance, it is possible to calculate a dose adjustment according to the so-called Dettli rules . The following applies in renal insufficiency (NI) versus normal kidney function (N):
- Dettli rule 1: Lower the maintenance dose of the drug by the factor of the individual excretion capacity (Q)
- or
- Dettli rule 2: Extension of the dosing interval by the factor
- or
- a combination of rule 1 and rule 2
When these rules are applied, exposure to the drug (AUC, average concentration) comparable to that of healthy kidneys can be expected in patients with renal insufficiency. In deciding whether any of these rules (and if so, which ones) are appropriate for a drug, the pharmacodynamics of that drug should be considered. Both rules lead to an AUC as in healthy kidneys. If Dettli rule 1 is applied, however, lower peak levels and higher trough levels are to be expected. When applying Dettli rule 2, the usual peak and valley levels are to be expected, but at (often significantly) greater intervals.
If a comparable exposure to the drug (AUC, average concentration) does not appear useful, other rules for dose adjustment should be considered. This could be the case, for example, if frequent peak levels at normal levels are necessary for a therapeutic effect and no clinically relevant side effects are expected due to an increased AUC.
literature
- F. Keller, T. Frankewitsch, D. Zellner, S. Simon: Unifying concept of pharmacokinetics derived from drug distribution and elimination in renal failure. In: Int J Clin Pharmacol Therap. Volume 33, 1995, pp. 546-549. PMID 8574504 .
- B. Hartmann, D. Czock, F. Keller: Drug therapy in patients with chronic renal failure. In: Dtsch Arztebl Int. Volume 107, No. 37, Sep 2010, pp. 647-655. PMID 20959896 .
swell
- ↑ a b L. Dettli: The kidney in pre-clinical and clinical pharmacokinetics. In: Jpn J Clin Pharmacol Ther. Volume 15, 1984, pp. 241-254.
Web links
- DOSING: Dose adjustment in case of renal insufficiency, Heidelberg University Hospital
- Pharmacology Toxicology University Hospital Basel DoseAdapt
- THG Lexicon ( Memento from September 28, 2007 in the Internet Archive ) Dose adjustment in case of renal insufficiency



