Fujimoto-Belleau reaction
The Fujimoto-Belleau reaction is a name reaction in organic chemistry. It is named after the two chemists George I. Fujimoto and Bernard Belleau and is used for the synthesis of α-substituted α, β- enones .
Overview reaction
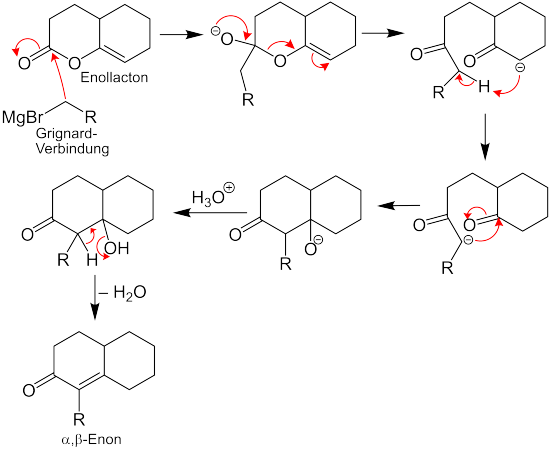
The reaction makes it possible, with the aid of a Grignard compound , to synthesize α-substituted α, β- enones from cyclic enol lactones .
Reaction mechanism
In the proposed reaction mechanism, the enol lactone reacts via various intermediate stages to form the α, β-enone:
application
An application of the Fujimoto-Belleau reaction is shown below. The methyl group (CH 3 ) that is crucial for the reaction is marked in blue :
Individual evidence
- ↑ a b Zerong Wang: Comprehensive organic name reactions and reagents . John Wiley, Hoboken, NJ 2009, ISBN 978-0-470-63885-9 , pp. 1155-1157 , doi : 10.1002 / 9780470638859.conrr42 .