Furanolactones
Furanolactones are chemical compounds from the class of natural substances . They have both a furan ring as a residue and the functional group of the lactones .
Representative
The compounds of the furanolactones occur exclusively in very complex chemical structures. Well-known representatives of this class of substances are:
- the salvinorines , such as B. Salvinorin A
- Columbin
- the limonoids , such as B. Limonine

|

|
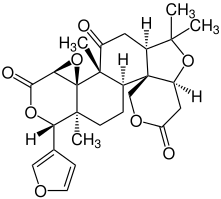
|
|---|---|---|
| Salvinorin A | Columbin | Limonine |
Occurrence
Like many natural substances, these compounds are found in the components of numerous plants. For example, limonin occurs in orange pits and columbin is part of the Calumbae Radix .
Individual evidence
- ↑ WW Harding, M. Schmidt, K. Tidgewell, P. Kannan, KG Holden, CM Dersch, RB Rothman, TE Prisinzano: Synthetic studies of neoclerodane diterpenes from Salvia divinorum: selective modification of the furan ring , Bioorganic & Medicinal Chemistry Letters, No. 16, 2006, pp. 3170-3174, doi: 10.1016 / j.bmcl.2006.03.062 .
- ↑ G. Koller, H. Czerny: About limonin, the bitter substance in orange pits. In: Monthly books for chemistry and related parts of other sciences . , No. 67, 1936, pp. 248-268, doi: 10.1007 / BF0271602 .
- ↑ H. Kohno, M. Maeda, M. Tanino, Y. Tsukio, N. Ueda, K. Wada, S. Sugie, H. Mori, T. Tanaka: A bitter diterpenoid furanolactone columbin from Calumbae Radix inhibits azoxymethane-induced rat colon carcinogenesis , Acta Crystallographica Section C, No. 45, 1989, pp. 300-303, doi: 10.1016 / s0304-3835 (02) 00159-3 .
- ↑ K. Swaminathan, UC Sinha, S. Ramakumar, RK Bhatt, BK Sabate: Structure of columbin, a diterpenoid furanolactone from Tinospora cordifolia Miers , Cancer Letters , No. 138, 2002, pp. 131-139, doi: 10.1107 / s0108270188010583 .