Hull cell
A Hull cell is a standardized electroplating system reduced to a laboratory scale (named after RO Hull). It consists of a trapezoidal container made of non-conductive material in which the electrodes are arranged in such a way that cathodic or anodic effects can be observed over a wide current density range.
The investigation of galvanic baths with the Hull cell has long been one of the standard methods of electroplating . With the Hull cell, the influences of the bath parameters (e.g. temperature, pH value, electrolyte composition, lack or excess of additives, cleanliness, contamination by foreign metals) on the properties of the deposited layer can be determined depending on the current density. For example, a new electrolyte can be tested in advance on a small scale.
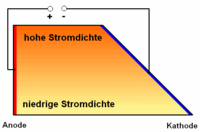
Due to the trapezoidal outline of the Hull cell, the distance between anode and cathode varies . As a result, the current density between cathode and anode is also subject to a variation, the smallest distance between the two electrodes corresponding to the highest current density and the smallest current density corresponding to the greatest distance between the two electrodes. However, the current density distribution is not linear, but increases faster as the distance becomes smaller. The anode material is selected according to the electrolyte to be tested (e.g. pure copper for copper electrolytes). The cathode, on the other hand, consists of a test sheet (usually brass or steel).
The evaluation of a test with the Hull cell is first carried out by describing and interpreting the metal coating on the test sheet according to its appearance (glossy, matt, stained, porous, rough ...). Statements about the throwing power of an electrolyte (galvanic bath) can be derived by measuring the layer thickness in the high and low current density range and forming a factor. Compare: Haring-Blum cell .
Since many electrolytes require a working temperature above room temperature, a Hull cell is usually heated. A heating plate (controlled by a thermal sensor in the cell) or a water bath can be used for this. Today, a hot plate with an integrated magnetic stirrer is often used, which ensures sufficient movement of the electrolyte.
literature
- Electroplating test with the Hull cell , DIN 50 957 Jan 1978
- C. Andrle, TW Jelinek, Hull cell for the investigation of galvanic electrolytes, Eugen G. Leuze, ISBN 3-87480-224-8
- Surface treatment, metallic and other inorganic coatings EN 12508: 2000
- Nohse, Walter The investigation of galvanic baths in the Hull cell , Saulgau, Eugen G. Leuze, ISBN 3-87480-016-4
- Hull, RO Current Density Range Characteristics, their Determination and Application , Proc. At the. Electroplater's Soc. 27 (1939)