Calorimetry
The Calorimetry refers to the measurement of quantities of heat which are coupled to biological, chemical or physical processes, and may be both exothermic and endothermic. A calorimeter is used for the determination . A distinction is made between direct and indirect calorimetry . This science was founded in 1756 by Joseph Black , professor of physics at the University of Glasgow and student of William Cullen , the inventor of the first, still purely experimental, ice machine.
Direct calorimetry
With direct calorimetry , the amount of heat is determined using a calorimeter . This method is also suitable for measuring the energy expenditure of an organism.
Historical animal calorimetry
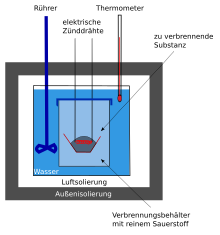
The first such animal calorimeter was built by Antoine Laurent de Lavoisier (see picture). The calorimeter vessel designed for a guinea pig represents the core of a sphere which was surrounded by two double-walled bowls. Both bowls were filled with pieces of ice. The outer shell served to isolate the device and make the inside a closed system. The heat entering the device from the outside is required to melt the ice in the outer shell. In this way, the partition between the outer and inner shell remains constant at 0 ° C as long as there is still ice in the outer shell. The ice in the inner shell is then used to measure the heat generation of the animal. The amount of heat generated by the animal is required to melt the ice. The melt water flows off at 0 ° C. The amount of melt water that has flowed out multiplied by the heat of melt serves as a measure of the heat given off by the animal. The prerequisite here is that the animal's body heat remains constant.
Indirect calorimetry
With indirect calorimetry , the amount of heat released is calculated indirectly via the measured oxygen consumption. From the amount of oxygen that z. B. a reaction or an organism consumed, and the equivalent (e.g. oxycaloric equivalent in metabolic reactions) can be calculated back to the heat released. This is particularly useful for large living beings such as B. to humans .
Gas calorimetry
The Gaskalorimetrie is a method for determining the calorific value of a gas by combustion of a gas sample.
The gas calorimeters are divided into the following classes according to the procedures used:
- Burning a gas sample in a calorimetric bomb ,
- Combustion of a gas in the open flame of a gas burner ,
- Combustion without a flame on a catalytic converter .
literature
- Franz Xaver Eder : Thermal and caloric properties . In: Working methods of thermodynamics . tape 2 . Springer Verlag, Berlin / Heidelberg 1983, ISBN 3-540-11727-X , 5th calorimetry, p. 119–395 , doi : 10.1007 / 978-3-642-93226-7 (calorimetric measurement of human heat production is dealt with in 5.6.5 Heat transfer calorimeters).
- Stefan Silbernagl , Agamemnon Despopoulos : Pocket Atlas Physiology. 7th, completely revised and expanded edition. Thieme, Stuttgart et al. 2007, ISBN 978-3-13-567707-1 .
- Ulrich Wernekinck (Ed.): Gas measurement and gas billing. 3. Edition. Vulkan-Verlag, Essen 2005, ISBN 3-8027-5617-7 .
Individual evidence
- ↑ Wolf D. Keidel (Ed.): Kurzgefaßtes Lehrbuch der Physiologie. 4th, revised and expanded edition. Thieme, Stuttgart 1975, ISBN 3-13-358604-1 , pp. 7-2.