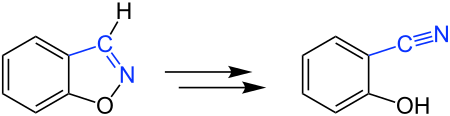
Kemp elimination
The Kemp elimination is a name reaction in organic chemistry . It is the base-catalyzed ring opening of a benzisoxazole . The reaction was named after the American chemist Daniel S. Kemp , who presented it for the first time in 1973.
Overview reaction
Cleavage of the N – O bond leads to ring opening of the benzisoxazole. The reaction takes place in a basic environment, so that it is accompanied by deprotonation. A phenol derivative is obtained as the product .
Reaction mechanism
The following reaction mechanism is described in the literature as follows:
An unstable intermediate stage 2 is initially formed from benzisoxazole 1 by deprotonation with a base . The pH value is then brought to 1 by adding hydrochloric acid , so that protonation occurs. The phenol derivative 3 is obtained as the product .
scope of application
The Kemp elimination is used as a model for many studies of enzyme-catalyzed reactions involving proton transfers. The reason for this is that it is a very simple reaction that takes place in one step. She is also very sensitive to changes in the medium.
Individual evidence
- ^ A b c Zerong Wang: Comprehensive Organic Name Reactions and Reagents , Wiley, 2010, ISBN 9780470638859 , pp. 1605-1607, doi: 10.1002 / 9780470638859 .
- ^ DS Kemp and Martha L. Casey: Physical organic chemistry of benzisoxazoles. II. Linearity of the Broensted free energy relation for the base-catalyzed decomposition of benzisoxazoles , Journal of the American Chemical Society , 1973, pp. 6670-6680, doi: 10.1021 / ja00801a024 .
- ^ H. Schulman and E. Keinan: Catalysis of the Kemp Elimination by Natural Coals , Organic Letters , 2000, pp. 3747-3750, doi: 10.1021 / ol000137u .