Kornblum's rule
The Kornblum rule is a concept in organic chemistry that allows the preferred product of a nucleophilic substitution with ambident nucleophiles to be estimated . The rule is based on the concept of hard and soft acids and bases ( HSAB concept ). In the case of an S N 1 reaction, the harder (more electronegative ) position of the nucleophile attacks, whereas in an S N 2 reaction, the softer (more nucleophilic) part is attacked.
example
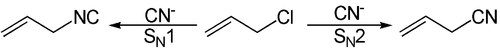
The Kornblum rule does not apply to all reactions with ambident nucleophiles, it is only an estimate of which main product and which by-product are formed. In the following example, the cyanide - anion | N≡C | - as a nucleophile, however, it is fulfilled:
| Nitrogen (N) | 3.04 |
| Carbon (C) | 2.55 |
This means that in an S N 1-like reaction the nitrogen attacks and in an S N 2-like reaction the carbon attacks .
This results in the following S N 1 reaction: CH 2 = CH-CH 2 -Cl + KCN, preferably the isonitrile compound CH 2 = CH-CH 2 -NC, while the likewise conceivable nitrile compound CH 2 = CH-CH 2 -CN is only produced as a by-product.
It should be noted that the direction of the reaction depends on the reaction conditions. Polar aprotic solvents favor S N 2 reactions, because here the hardly solvated anions can fully play out their nucleophilicity, while in protic solvents the solvated anions are hindered in their reactivity and so S N 1 reactions take place preferentially.
Cations can also influence the direction of the reaction: if KCN is replaced by AgCN in the above example, a higher yield of isonitrile is obtained because the silver ion, as a soft Lewis acid , coordinates preferentially to the (softer) carbon atom of the cyanide ion and thus prevents it from attack. This shifts the direction of the reaction to "S N 1-like". When alkyl halides react with silver nitrite, more alkyl nitrites are obtained than with alkali cyanides, since the silver ion coordinates to the - in this case softer - N atom. Furthermore, Ag + ions support the detachment of chloride, bromide and iodide by coordinating to the halogen, which increases the positive polarization of the neighboring carbon atom; if necessary, the complex dissociates to form the silver halide and the carbenium ion, in which case rearrangements can also be observed.
Individual evidence
- Author collective: Organikum . 21st edition, pp. 222-223, Wiley-VCH, 2001, ISBN 3-527-29985-8 .