Meyers aldehyde synthesis
The Meyers aldehyde synthesis , also Meyers synthesis , is a name reaction in organic chemistry that goes back to the US chemist Albert I. Meyers (1932-2007). The reaction was first described in 1969.
Overview reaction
A 1,3-oxazine is used as the starting material in the Meyers aldehyde synthesis:
Reaction mechanism
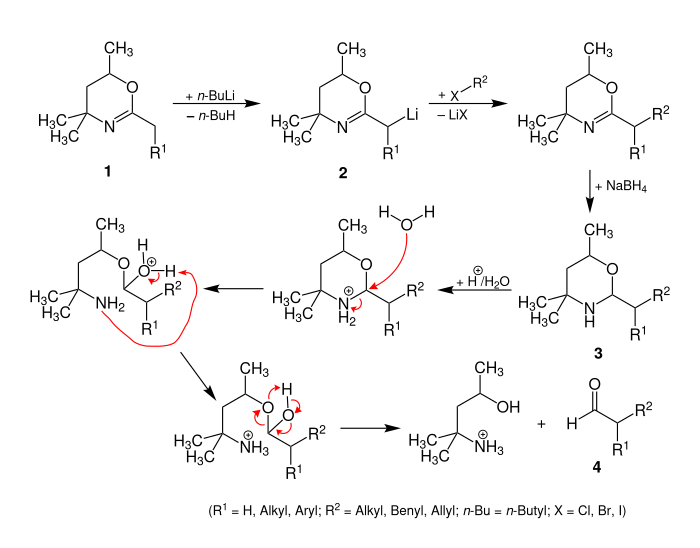
A possible reaction mechanism for the Meyers aldehyde synthesis is described by Zerong Wang. The starting compound is a dihydro-1,3-oxazine 1 with an alkyl group in the 2-position. The α- proton of the alkyl radical is CH-acidic due to the neighboring heteroatoms and is deprotonated by strong bases (e.g. butyllithium) to form the lithium compound 2 . Subsequent electrophilic alkylation with an alkyl halide R 2 -X and reduction of the heterocycle lead to the tetrahydrooxazine 3 .
Since 3 is a hemiaminal , a substituted aldehyde 4 is formed during acid hydrolysis .
criticism
From the point of view of atom economy , the Meyers aldehyde synthesis is one of the less efficient reactions because, in addition to the target molecule (aldehyde 4 ), considerable amounts of waste (including alkali halides ) are produced in at least stoichiometric proportions. Therefore the application is limited to the laboratory scale.
Individual evidence
- ^ Albert I. Meyers, Aiko Nabeya, H. Wayne Adickes, Ieva R. Politzer: Aldehydes from dihydro-1,3-oxazines. I. Synthesis of aliphatic aldehydes and their C-1 deuterated derivatives , J. Am. Chem. Soc. , 1969 , 91 (3), pp. 763-764, doi : 10.1021 / ja01031a053 .
- ^ Albert I. Meyers, Aiko Nabeya, H. Wayne Adickes, J. Michael Fitzpatrick, G. Ray Malone, Ieva R. Politzer: Aldehydes from dihydro-1,3-oxazines. II. Synthesis of α, β-unsaturated aldehydes and their C-1 deuterated derivatives , J. Am. Chem. Soc. , 1969 , pp. 764-765, doi : 10.1021 / ja01031a054 .
- ^ Albert I. Meyers, H. Wayne Adickes, Ieva R. Politzer, Warren N. Beverung: Aldehydes from dihydro-1,3-oxazines. III. Synthesis of cycloalkanecarboxaldehydes , J. Am. Chem. Soc. , 1969 , pp. 765-767, doi : 10.1021 / ja01031a055 .
- ^ Ieva R. Politzer, AI Meyers: Aldehydes from 2-Benzyl-4,4,6-Trimethyl-5,6-Dihydro-1,3 (4H) -Oxazine: 1-Phenylcyclopentanecarboxaldehyde In: Organic Syntheses . 51, 1971, p. 24, doi : 10.15227 / orgsyn.051.0024 ; Coll. Vol. 6, 1988, p. 905 ( PDF ).
- ↑ a b c Zerong Wang: Comprehensive Organic Name Reactions and Reagents, Volume 2 . John Wiley, Hoboken (NJ) 2009, ISBN 978-0-470-28662-3 , pp. 1913-1916 .