Pictet-Gams synthesis
The Pictet-Gams synthesis , named after the Swiss chemists Amé Pictet (1857–1937) and Alfons Gams (1884–1955), is a name reaction in organic chemistry and was first described in 1910. The Pictet-Gams synthesis enables the synthesis of isoquinoline derivatives from N - (2-hydroxy-2-phenylethyl) amides.
Overview reaction
In the Pictet-Gams synthesis, an isoquinoline derivative is formed from an N - (2-hydroxy-2-phenylethyl) amide by adding a drying agent such as phosphorus pentoxide or phosphorus oxychloride with double dehydration:
Instead of the hydroxyl group , a methoxy or ethoxy group can also be present in the educt. In this case, methanol or ethanol is also split off in addition to water.
Reaction mechanism
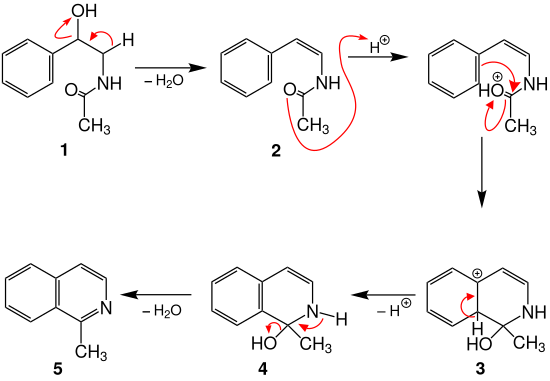
Various reaction mechanisms are suggested in the literature. A possible reaction mechanism that can be found in the literature is illustrated using N - (2-hydroxy-2-phenylethyl) acetamide ( 1 ) as an example :
In the first step, water is split off from the amide 1 and the intermediate product 2 is formed. After protonation of the carbonyl oxygen atom, cyclization occurs with the elimination of aromaticity; the mesomeric stabilized carbenium ion 3 is formed. Intermediate stage 4 is formed by deprotonation with rearomatization . The product 1-methylisoquinoline ( 5 ) is then formed by renewed dehydration.
Individual evidence
- ↑ GND 127461795
- ↑ Amé Pictet, Alfons Gams: About a new method for the synthetic representation of the isoquinoline bases. In: Reports of the German Chemical Society . 43 (2), 1910, pp. 2384-2391, doi: 10.1002 / cber.191004302206 .
- ↑ a b c Zerong Wang: Comprehensive Organic Name Reactions and Reagents. Wiley, 2010, ISBN 978-0-471-70450-8 , pp. 2206-2209.
- ^ Jie Jack Li: Name Reactions. A Collection of Detailed Reaction Meachanisms. Springer International Publishing, 2014, ISBN 978-3-319-03978-7 , p. 478.