Chelatometry
The chelatometry (v. Gr. Chele "crab claw"), even complexometry called, is a method of analytical chemistry . It is mostly used for the quantitative determination of metal in aqueous solution by titration . It was developed in 1945 by Gerold Schwarzenbach . It is based on the formation of chelate complexes , wrapping complexes with multidentate ligands , from metal ions and complexing agents .
An indicator is added to the solution to be titrated . In the free state, this shows a different color than after binding to the metal ion. Since the indicator is only weakly bound to the metal ion, it is released when the chelating ligand is added, so that the color of the solution changes. A buffer solution is often used to keep the pH constant. The metal indicator and buffer can also be combined in indicator-buffer tablets .
In trading, chelating agents found, for example, under the names complexone , Idranal or titriplex followed by a Roman numeral.
| Complexone | Common name | abbreviation | Structural formula |
|---|---|---|---|
| I. | Nitrilotriacetic acid | NTA |

|
| II | Ethylenediaminetetraacetic acid | EDTA |

|
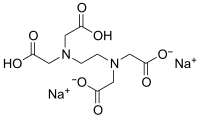
| III | Ethylenediaminetetraacetic acid disodium salt | EDTA-Na 2 |
|
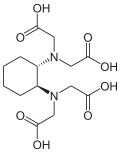
| IV | Cyclohexanediaminetetraacetic acid | CDTA |
|
| V | Diethylenetriaminepentaacetic acid | DTPA |

|
| VI |
Ethylene glycol bis (aminoethyl ether) - N, N, N ', N'-tetraacetic acid |
EGTA |

|
| VII | N- (2-Hydroxyethyl) ethylenediamine-N, N, N'-triacetic acid trisodium salt | HEDTA |

|
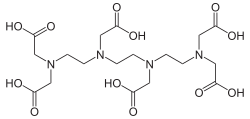
| VIII | Triethylenetetraminehexaacetic acid | TTHA |
|
Individual evidence
- ↑ Schwarzenbach, G. & Flaschka, H. (1965): The complexometric titration. Ferdinand Enke Verlag, Stuttgart.
- ↑ G. Schwarzenbach, E. Kampitsch and R. Steiner (1945): Complexone I. About the salt formation of nitrilotriacetic acid. In: Helvetica Chimica Acta. Vol. 28, No. 1, pp. 828-840. doi : 10.1002 / hlca.194502801121