Electrodialysis
The electrodialysis (ED) (from the Greek dialysis = resolution) is an electrochemically driven membrane process (see Membrane Technology ) in the ion exchange membranes in combination with an electric potential difference can be used to separate ionic species of uncharged solvents or impurities.
Working principle
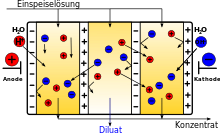
In an electrodialysis separator, the space between two electrodes is separated by a stack of alternating anion and cation exchange membranes. Each pair of ion exchange membranes forms a separate “cell”. In technical systems, these stacks consist of more than two hundred membrane pairs. If an electrical direct voltage is applied to the electrodes, the anions migrate to the anode. The anions can simply pass the positively charged anion exchange membrane, but they are each stopped at the nearest negatively charged cation exchange membrane. Because the same thing happens (of course with the opposite sign) with the cations , the net effect of electrodialysis is an accumulation of salts in the cells with an odd number (anion exchange membrane / cation exchange membrane), while the cells with an even number (cation exchange membrane / anion exchange membrane) are depleted in salt . The solutions with increased salt concentration are combined to form the concentrate , while the low-salt solutions form the diluate .
Because of the large number of cells between the electrodes, electrochemical electrode reactions have practically no influence on the energy consumption of an electrodialysis separator. Taking into account the energy expenditure for pumping the solutions through the separator units, the energy expenditure for electrodialysis separations is proportional to the salt concentration of the input solution. For this reason, the electroosmosis is more economical than z. B. the reverse osmosis. The electrodialysis process currently competes strongly with reverse osmosis systems for desalination of brackish water. In Japan, around 350,000 tons of table salt are produced annually with the help of electrodialysis systems. Sea water is enriched from about 15 g / L to 200 g / L sodium chloride content before the residual water is removed by conventional evaporation. A pilot plant for the desalination of seawater in Singapore processes 50 cubic meters of water a day and only needs 1.5 kilowatt hours of electricity per cubic meter. The most efficient desalination technology to date, reverse osmosis, costs more than twice as much energy. However, as the salt concentration drops, the process becomes inefficient because the electrical resistance of the water increases. The last percent of salt is extracted by continuous electrodeionization . Ion exchange resins take up the ions between the membranes and transport them on.
Other technically realized applications of electrodialysis relate to wastewater treatment , e.g. B. the regeneration of copper plating baths in the production of integrated circuits. Cheese whey is demineralized by means of electrodialysis and dextran solutions are desalinated using this method.
The technical use of electrodialysis processes is restricted by the limited selective permeability of currently available ion exchange membranes at high salt concentrations. The process becomes uneconomical as soon as the electricity utilization falls below 50–60%. With today's ion-selective membranes, this happens when the salt concentration exceeds approx. 10–15 mol / L.
Electrodialysis with pole reversal
This type of electrodialysis, or UED from reverse electro-dialysis or English: Electrodialysis Reversal and EDR for short , works with a regular change in the electrical polarity of the membranes. This means that the outlay on equipment is significantly higher, but the reversal of the pole from the anode to the cathode results in internal cleaning of the membranes. This cleaning effect is achieved because the pH value drops on the active cathode surface of the membrane and increases on the anode surface. This suppresses the formation of deposits even in slightly oversaturated water. The EDR process is used particularly successfully in the production of drinking water from more salty raw water.
Reverse electrodialysis
The reverse electrodialysis is the process in which a salt solution and fresh water of alternating cathode and anode exchange membranes are passed through a block. The difference in chemical potential between salt water and fresh water creates a voltage difference across each membrane. The total electrical potential of the system corresponds to the sum of the potential differences of all membranes.
See also
literature
- H. Strathmann: Electrodialysis and related processes . In: RD Noble, SA Stern (eds.), Membrane separations technology - Principles and applications , pp. 415-465, Elsevier, Amsterdam 1995.
- E. Korngold, K. Kock, H. Starthmann, Desalination 24, 129-139 (1978)
- H. Strathmann, B. Bauer, HJ Rapp, CHEMTECH 1993, 17-24.
- R. Köhler, R. Brunner, WLB Water, Air and Soil, 7–8 / 1991
Individual evidence
- ↑ IDW-Online: Desalination of sea water
- ^ Rudolf Kohler, Rudolf Brunner; The reverse dialysis process for water and wastewater desalination; in: WLB Wasser, Luft und Boden , 1991, Heft 7-8, pp. 22-26
- ↑ Chr. Von Oppel; Water desalination with electrochemical membrane processes; in: VGB Kraftwerkstechnik , Volume 66, 1986
- ↑ University of Twente : Power generation by reverse electrodialysis ( Memento of the original of September 27, 2008 in the Internet Archive ) Info: The archive link was automatically inserted and not yet checked. Please check the original and archive link according to the instructions and then remove this notice. August 2007