Von Braun reaction
The Von Braun reaction is a name reaction in organic chemistry . It was named after its discoverer, the German chemist Julius von Braun (1875–1939). In contrast to the Von Braun degradation , this reaction is used to synthesize substituted cyanamides from tertiary amines . The cynanamides obtained can then be converted into secondary amines by hydrolysis . The Von Braun reaction is therefore suitable, for example, for the production of secondary amines from tertiary amines.
Overview reaction
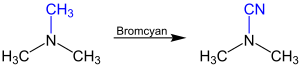
The Von Braun reaction is used to substitute one of the alkyl or aryl groups of a tertiary amine . As a new substituent by means of cyanogen bromide, a cyano group introduced. The following reaction equation illustrates this using the example of the reaction of trimethylamine to dimethylcyanamide:
Reaction mechanism
A possible reaction mechanism for the Von Braun reaction according to the overview reaction is described below:
First, the trimethylamine reacts with the cyanogen bromide to take up a cyano group. This produces a quaternary ammonium salt which, in the next step, reacts to dimethylcyanamide by splitting off bromomethane . This is a second-order nucleophilic substitution ( S N 2 ).
Sample reaction
The following reaction scheme shows an example of the use of the Von Braun reaction to convert a tertiary to a secondary amine. The product of the reaction is the drug fluoxetine , which is used as an antidepressant :
Atomic economy
Since this reaction is a substitution reaction, one hundred percent atomic efficiency is excluded. It should be borne in mind that cyanogen bromide is used in stoichiometric amounts in the Von Braun reaction . In addition, a bromoalkane is also obtained in stoichiometric amounts as waste material. The atom economy of this reaction is accordingly greater, the smaller the molecular mass of the substituted alkyl group and the greater the overall molar mass of the tertiary amine used.
Individual evidence
- ↑ Julius von Braun: The splitting of cyclic bases by cyanogen bromide . In: Reports of the German Chemical Society . tape 40 , no. 3 , 1907, pp. 3914-3933 , doi : 10.1002 / cber.190704003198 .
- ↑ a b Jie Jack Li: Name reactions: A collection of detailed mechanisms and synthetic applications . 5th ed.Springer, Cham 2014, ISBN 978-3-319-03979-4 , pp. 619 , doi : 10.1007 / 978-3-319-03979-4 .
- ↑ Michael Freissmuth, Stefan Offermanns, Stefan Böhm: Pharmacology & Toxicology: From the Molecular Basics to Pharmacotherapy . Springer, Berlin, Heidelberg 2012, ISBN 978-3-642-12353-5 , pp. 283-294 .