Kavapyrone
Kavapyrone or Kavalactone are a group of pharmacologically active, organic compounds that occur in the kava plant. By 2006, 18 different kavapyrons had been identified.
properties
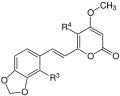
All kavapyrones are substituted, six-membered lactones , so-called styryl-alpha-pyrones. They are divided into two groups, the enolid pyrons and the dienolid pyrons. In the first group, the lactone ring contains one double bond , in the second there are two double bonds. Common to all kavapyrons is a β- methoxy group in the lactone ring:
All kavapyrons are crystalline in their purest form, very sparingly soluble in water, but easily soluble in non-polar solvents. They are all closely related chemically and therefore tend to form mixed crystals . The representation in pure form is associated with a lot of effort.
The most important representatives of this group of substances are: Kavain, Dihydrokavain, Methysticin, Dihydromethysticin, Yangonin and Desmethoxyyangonin.
| Surname | framework | R 1 | R 2 | R 3 | R 4 |
|---|---|---|---|---|---|
| Yangonin | 1 | -OCH 3 | -H | -H | -H |
| 10-methoxyyangonine | 1 | -OCH 3 | -H | -OCH 3 | -H |
| 11-methoxyyangonine | 1 | -OCH 3 | -OCH 3 | -H | -H |
| 11-hydroxyyangonine | 1 | -OCH 3 | -OH | -H | -H |
| 5,6-dehydrocavaine | 1 | -H | -H | -H | -H |
| 11-methoxy-12-hydroxydehydrokavaine | 1 | -OH | -OCH 3 | -H | -H |
| 7,8-dihydroyangonine | 2 | -OCH 3 | -H | -H | -H |
| Kavain | 3 | -H | -H | -H | -H |
| 5-hydroxykavain | 3 | -H | -H | -H | -OH |
| 5,6-dihydroyangonine | 3 | -OCH 3 | -H | -H | -H |
| 7,8-dihydrocavaine | 4th | -H | -H | -H | -H |
| 5,6,7,8-tetrahydroyangonine | 4th | -OCH 3 | -H | -H | -H |
| 5,6-dehydromethysticin | 5 | -O-CH 2 -O- | -H | -H | |
| Methysticin | 7th | -O-CH 2 -O- | -H | -H | |
| 7,8-dihydromethysticin | 8th | -O-CH 2 -O- | -H | -H | |
- Scaffolding of the Kavalactones
Extraction and presentation
Pharmaceutically usable extracts are obtained from the kava plant by extraction with a suitable solvent mixture . With ethanol / water one obtains extracts with a maximum of 30% of kavapyrone, with acetone / water extracts with about 70%.
The synthesis of the kavapyrons is possible using various methods. The synthesis of rac -dihydropyrones succeeds under mild conditions by first allowing two equivalents of bromozinc acetate to react with the appropriate aldehyde in the presence of TMEDA by means of a double Reformatzki reaction , in order to subsequently bring about the ring closure by treatment first with sodium hydroxide solution, then with hydrochloric acid. Dimethyl sulfate can be used as a reagent for the final O- methylation .
literature
- A. Ligresti, R. Villano, M. Allarà, I. Ujváry, V. Di Marzo: Kavalactones and the endocannabinoid system: the plant-derived yangonin is a novel CB1 receptor ligand. In: Pharmacological research. Volume 66, Number 2, August 2012, pp. 163-169, doi : 10.1016 / j.phrs.2012.04.003 . PMID 22525682 .
- R. Teschke: Kava hepatotoxicity - a clinical review. In: Annals of hepatology. Volume 9, Number 3, 2010 Jul-Sep, pp. 251-265, PMID 20720265 (review).
- YM Tzeng, MJ Lee: Neuroprotective properties of kavalactones. In: Neural regeneration research. Volume 10, number 6, June 2015, pp. 875-877, doi : 10.4103 / 1673-5374.158335 , PMID 26199594 , PMC 4498339 (free full text).
- F. Pantano, R. Tittarelli et al. a .: Hepatotoxicity Induced by "the 3Ks": Kava, Kratom and Khat. In: International journal of molecular sciences. Volume 17, number 4, 2016, doi : 10.3390 / ijms17040580 , PMID 27092496 , PMC 4849036 (free full text) (review).
Individual evidence
- ↑ R. Hänsel, P. Bähr, J. Elich: "Isolation and characterization of two previously unknown dyes of the kawa rhizome", in: Arch Pharm , 1961 , 294 (11), pp. 739-743; doi : 10.1002 / ardp.19612941108 .
- ↑ Masahiro Mineno M, Sawai Y, Kanno K, Sawada N, Mizufune H: A rapid and diverse construction of 6-substituted-5,6-dihydro-4-hydroxy-2-pyrones through double Reformatsky reaction . In: Tetrahedron . 69, No. 51, 2013, pp. 10921-10926. doi : 10.1016 / j.tet.2013.10.079 .