User:Mad Price Ball/Draft: Difference between revisions
→Regulation of replication: adding termination of replication section |
→Old material: delete covered material |
||
| Line 99: | Line 99: | ||
Rolling circle replication has found wide uses in academic research and biotechnology, and has been successfully used for amplification of DNA from very small amounts of starting material. |
Rolling circle replication has found wide uses in academic research and biotechnology, and has been successfully used for amplification of DNA from very small amounts of starting material. |
||
== Old material == |
|||
;Telomerase |
|||
{{main|Telomerase|Telomere}} |
|||
A unique problem that occurs during the replication of linear chromosomes is chromosome shortening. Chromosome shortening occurs when the primer at the 5' end of the lagging strand is degraded. Because DNA polymerase cannot add new nucleotides to the 5' end of DNA (there is no place for a new primer), the ends would shorten after each round of replication. However, in most replicating cells a small amount of telomerase is present, and this enzyme extends the ends of the chromosomes so that this problem does not occur. This extension occurs when the telomerase enzyme binds to a section of DNA on the 3' end and extends it using the normal replication machinery. This then allows for a primer to bind so that the complementary strand can be extended by normal lagging strand synthesis. Finally, telomeres must be capped by a protein to prevent chromosomal instability. |
|||
==== DNA replication in archaea ==== |
==== DNA replication in archaea ==== |
||
Revision as of 16:36, 3 March 2008
THIS IS NOT A WIKIPEDIA PAGE NOTE: This is not a wikipedia page, this is a user page used to work on drafts of pages. Madeleine
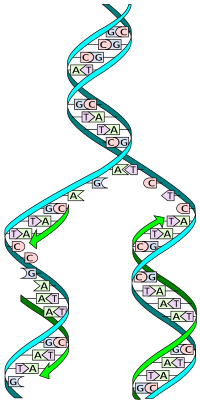
DNA replication is the process of copying a double-stranded DNA molecule to form two double-stranded molecules. The process of DNA replication is a fundamental process used by all living organisms as it is the basis for biological inheritance. As each DNA strand holds the same genetic information, both strands can serve as templates for the reproduction of the opposite strand. The template strand is preserved in its entirety and the new strand is assembled from nucleotides. This process is called semiconservative replication. The resulting double-stranded DNA molecules are identical; proofreading and error-checking mechanisms exist to ensure near perfect fidelity.
In a cell, DNA replication must happen before cell division can occur. DNA synthesis begins at specific locations in the genome, called "origins", where the two strands of DNA are separated. RNA primers attach to single stranded DNA and DNA polymerase extends from the primers to form new strands of DNA, adding nucleotides matched to the template strand. The unwinding of DNA and synthesis of new strands forms a replication fork. In addition to DNA polymerase, a number of enzymes are associated with the fork and assist in the initiation and continuation of DNA synthesis.
DNA replication can also be performed artificially, using the same enzymes used within the cell. DNA polymerases and artificial DNA primers are used to initiate DNA synthesis at known sequences in a template molecule. The polymerase chain reaction (PCR), a common laboratory technique, employs artificial synthesis in a cyclic manner to rapidly and specifically amplify a target DNA fragment from a pool of DNA.
DNA structure
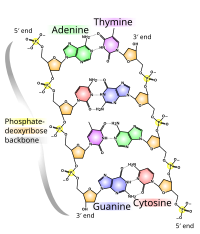
DNA usually exists in a double-stranded structure, with both strands coiled together to form the characteristic double-helix. Each single strand of DNA is a chain of four types of nucleotide: adenine, cytosine, guanine, and thymine. A nucleotide consists of a phosphate and a deoxyribose sugar — forming the backbone of the DNA double helix — plus a base that points inwards. Nucleotides are matched between strands through hydrogen bonds to form base pairs. Adenine pairs with thymine and cytosine pairs with guanine.
The physical pairing of bases in DNA means that the information contained within each strand is redundant — the nucleotides on a single strand can be used to reconstruct nucleotides on a newly synthesized partner strand.
DNA strands have a directionality, and the different ends of a single strand are called the "3' end" and the "5' end" (these refer to the carbon atom in ribose that the next phosphate in the chain attaches to).[1] In addition to being complementary, the two strands of DNA are antiparallel — they are oriented in opposite directions. This directionality has a consequences in DNA synthesis, because DNA polymerase can only synthesize DNA in one direction by adding nucleotides to the 3' end of a DNA strand.
DNA polymerase

DNA polymerases are a family of enzymes critical for all forms of DNA replication. A DNA polymerase synthesizes a new strand of DNA by extending the 3' end of an existing nucleotide chain, adding new nucleotides matched to the template strand one at a time. Some DNA polymerases may also have some proofreading ability, removing nucleotides from the end of a strand in order to remove any mismatched bases. DNA polymerases are generally extremely accurate, making less than one error for every million nucleotides added.
The energy for the process of DNA polymerization comes from the two additional phosphates attached to each of the unincorporated nucleotides — these free nucleotides, also known as nucleoside triphosphates, contain a total of three phosphates. When a nucleotide is being added to a growing DNA strand, two of the phosphates are removed and the energy produced is used to attach the remaining phosphate to the growing chain. The energetics of this process may also explain the directionality of synthesis - if DNA were synthesized in the 3' to 5' direction, the energy for the process would come from the 5' end of the growing strand rather than from free nucleotides. During proofreading, if the 5' nucleotide needed to be removed this triphosphate end would be lost, losing the energy source required to add a new nucleotide to the end.
DNA polymerase can only extend an existing DNA strand paired with a template strand, it cannot begin the synthesis of a new strand. To do this a short fragment of DNA or RNA, called a primer, must be created and paired with the template strand before DNA polymerase can synthesize new DNA.
DNA replication within the cell
Origins of replication
For a cell to divide, it must first replicate its DNA. This process is initiated at particular points within the DNA, known as "origins", which are targeted by proteins that separate the two strands and initiate DNA synthesis. Origins contain DNA sequences recognized by replication initiator proteins (eg. dnaA in E coli' and the Origin Recognition Complex in yeast).[2] These initiator proteins recruit other proteins to separate the two strands and initiate replication forks.
Initiator proteins recruit other proteins to separate the DNA strands at the origin, forming a bubble. Origins tend to be "AT-rich" (rich in adenine and thymine bases) to assist this process — because A-T base pairs have two hydrogen bonds (rather than the three formed in a C-G pair) strands rich in these nucleotides are generally easier to separate. Once strands are separated, RNA primers are created on the template strands and DNA polymerase extends these to create newly synthesized DNA.
As DNA synthesis continues, the original DNA strands continue to unwind on each side of the bubble, forming replication forks. In bacteria, which have a single origin of replication on their circular chromosome, this process eventually creates a "theta structure" (resembling the Greek letter theta: θ). In contrast, eukaryotes have longer linear chromosomes and initiate replication at multiple origins within these.
The replication fork
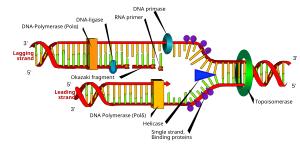
The replication fork is a structure which forms when DNA is being replicated. It is created through the action of helicase, which breaks the hydrogen bonds holding the two DNA strands together. The resulting structure has two branching "prongs", each one made up of a single strand of DNA.
- Leading strand synthesis
In DNA replication, the leading strand is defined as the new DNA strand at the replication fork that is synthesized in the 5'→3' direction in a continuous manner. When the enzyme helicase unwinds DNA, two single stranded regions of DNA (the "replication fork") form. On the leading strand DNA polymerase III is able to synthesize DNA using the free 3' OH group donated by a single RNA primer and continuous synthesis occurs in the direction in which the replication fork is moving.
- Lagging strand synthesis
The lagging strand is the DNA strand at the opposite side of the replication fork from the leading strand, running in the 3' to 5' diraction. Because DNA polymerase cannot synthesize in the 3'→5' direction, the lagging strand is synthesized in short segments known as Okazaki fragments. Along the lagging strand's template, primase builds RNA primers in short bursts. DNA polymerases are then able to use the free 3' OH groups on the RNA primers to synthesize DNA in the 5'→3' direction. The RNA fragments are then removed (different mechanisms are used in eukaryotes and prokaryotes) and new deoxyribonucleotides are added to fill the gaps where the RNA was present. DNA ligase then joins the deoxyribonucleotides together, completing the synthesis of the lagging strand.
- Dynamics at the replication fork
As helicase unwinds DNA at the replication fork the DNA ahead is forced to rotate — this process results in a build-up of twists in the DNA ahead. This build-up would form a resistance that would eventually halt the progress of the replication fork. DNA topoisomerases are enzymes that solve these physical problems in the coiling of DNA. Topoisomerase I cuts a single backbone on the DNA, allowing the strands to swivel around each other to remove the build-up of twists. Topoisomerase II cuts both backbones, allowing one double-stranded DNA to pass through another, thereby removing knots and entanglements that can from within and between DNA molecules.
Bare single-stranded DNA has a tendency to fold back upon itself and form secondary structures; these structures can interfere with the movement of DNA polymerase. To prevent this, single-strand binding proteins bind to the DNA until a second strand is synthesized, preventing secondary structure formation.
Clamp proteins form a sliding clamp around DNA, helping the DNA polymerase maintain contact with its template and thereby assisting with processivity. The inner face of the clamp allows DNA to be threaded through it. Once the polymerase reaches the end of the template or detects double stranded DNA, the sliding clamp undergoes a conformational change which releases the DNA polymerase. Clamp-loading proteins are used to initially load the clamp, recognizing the junction between template and RNA primers.
Regulation of replication

- Eukaryotes
Within eukaryotes, DNA replication is controlled within the context of the cell cycle. As the cell grows and divides, it progresses through stages in the cell cycle; DNA replication occurs during the S phase (Synthesis phase). The progress of the eukaryotic cell through the cycle is controlled by cell cycle checkpoints. Progression through checkpoints is controlled through complex interactions between various proteins, including cyclins and cyclin-dependent_kinases.
The G1/S checkpoint (or restriction checkpoint) regulates whether eukaryotic cells enter the process of DNA replication and subsequent division. Cells which do not proceed through this checkpoint are quiescent in the "G0" stage and do not replicate their DNA.
Replication of chloroplast and mitochondrial genomes occurs independent of the cell cycle, through the process of D-loop replication.
- Bacteria
Bacteria do not go through a well defined cell cycle and instead continuously copy their DNA, resulting in multiple and fractional copies of the genome within any given cell. Within E coli, the most well characterized bacteria, regulation of DNA replication can be achieved through several mechanisms, including: the hemimethylation and sequestering of the origin sequence, the ratio of ATP to ADP, and the levels of protein DnaA. These all control the process of initiator proteins binding to the origin sequences.
Because E coli methylates GATC DNA sequences, DNA synthesis results in hemimethylated sequences. This hemimethylated DNA is recognized by a protein (SeqA) which binds and sequesters the origin sequence; in addition, dnaA (required for initiation of replication) binds less well to hemimethylated DNA. As a result, newly replicated origins are prevented from immediately initiating another round of DNA replication.
ATP builds up when the cell is in a rich medium, triggering DNA replication once the cell has reached a specific size. ATP competes with ADP to bind to DnaA, and the DNA-ATP complex is able to initiate replication. A certain number DnaA proteins are also required for DNA replication — each time the origin is copied the number of binding sites for DnaA doubles, requiring the synthesis of more DnaA to allow for another initiation of replication.
Termination of replication
Because bacteria have circular chromosomes, termination of replication occurs when the two replication forks meet each other on the opposite end of the parental chromosome. E coli regulate this process through the use of termination sequences which, when bound by the Tus protein, allow only one direction of replication fork to pass through — as a result, the replication forks are contrained to always meet within the termination region of the chromosome.
Eukaryotes initiate DNA replication at multiple points in the chromosome, so replication forks meet and terminate at many points in the chromosome; these are not known to be regulated in any particular manner. Because eukaryotes have linear chromosomes DNA replication often fails to synthesize to the very end of the chromosomes (telomeres), resulting in the problem of telomere shortening. To counter this loss the enzyme telomerase extends the repetitive sequences of the telomere region, thereby protecting the chromosome from loss of sequences at the ends.
Rolling circle replication
Replication of the bacterial chromosome is known as theta (θ) replication. However, another method of replication exists in bacterial cells, known as rolling circle replication.
Rolling circle replication describes a process of DNA replication that can rapidly synthesize multiple copies of circular molecules of DNA, such as plasmids and the genomes of bacteriophages.
Rolling circle replication is initiated by an initiator protein encoded by the plasmid or bacteriophage DNA. This protein is able to nick one strand of the double-stranded, circular DNA molecule at a site called the double-strand origin (DSO) and remains bound to the 5' phosphate end of the nicked strand. The free 3' hydroxyl end is released and can serve as a primer for DNA synthesis by DNA polymerase III. Using the unnicked strand as a template, replication proceeds around the circular DNA molecule, displacing the nicked strand as single-stranded DNA.
Continued DNA synthesis can produce multiple single-stranded linear copies of the original DNA in a continuous head-to-tail series. These linear copies can be converted to double-stranded circular molecules through the following process: First, the initiator protein makes another nick to terminate synthesis of the first (leading) strand. RNA polymerase and DNA polymerase III then replicate the single-stranded origin (SSO) DNA to make another double-stranded circle. DNA polymerase I removes the primer, replacing it with DNA, and DNA ligase joins the ends to make another molecule of double-stranded circular DNA.
A striking feature of rolling circle replication is the uncoupling of the replication of the two strands of the DNA molecule. In contrast to common modes of DNA replication where both the parental DNA strands are replicated simultaneously, in rolling circle replication one strand is replicated first (which protrudes after being displaced, giving the characteristic appearance) and the second strand is replicated after completion of the first one.
Rolling circle replication has found wide uses in academic research and biotechnology, and has been successfully used for amplification of DNA from very small amounts of starting material.
DNA replication in archaea
You must add a |reason= parameter to this Cleanup template – replace it with {{Cleanup|section|reason=<Fill reason here>}}, or remove the Cleanup template.
Understanding DNA replication in the archeaota is just beginning, and it is the goal of this section to provide a basic understanding of how DNA replication occurs in these unique prokaryotes. In addition, this section aims to provide a comparison between the three domains.
- Origin of replication
The origins of archaea are AT rich, and generally have one or more AT stretches. In addition, long inverted repeats flank both ends of the origin, and are thought to be important in the initiation process and may serve a function similar to the DnaA boxes in the eubacteria. The genes that code for Cdc6/Orc1 are also located near the origin region, and this arrangement may allow these proteins to associate with the origin as soon as they are translated.
- Initiation
Initiation of replication begins with the binding of Cdc6/Orc1 to the origin in an ATP independent manner. This complex is constitutively expressed and most likely forms the origin binding proteins (OBP). Due to their similarity with proteins involved in eukaryotic initiation, Cdc6/Orc1 may be involved in helicase loading in archaea. However, other evidence suggests that this complex may function as an initiator and create a sufficiently large replication bubble to allow the helicase (Mcm) to load without the presence of a loader. Once loading of this complex is complete, however, the DNA melts, and helicase can be loaded.
In archaea, a hexameric protein known as the Mcm complex may function as the primary helicase. This protein is homologous to the eukaryotic Mcm complex. In archaea, there is no cdt1 homologue, and the helicase may be able to self-assemble at an archaeal origin without the need for a helicase loader. These proteins possess 3'->5' helicase capability.
- Elongation
Single stranded binding protein (SSB) prevents exposed single stranded DNA from forming any secondary structures or reannealing. This complex is able to recruit primase, DNA polymerase and other replication machinery. The mechanisms of this process are similar to those in eukaryotes.
- Similarities to eukaryotic and eubacterial replication
- ORC is homologous to Cdc6/Orc1 in archaea and may represent the ancestral state of the eukaryotic pre-RC.
- A homologous Mcm protein exists between eukarya and archaea
- The structure of Cdc6/Orc1 resembles the tertiary structure of DnaA in eubacteria
- Both eukaryotic and archaeal helicases possess 3'->5' helicase capability
- Archaeal SSB is similar to RPA
See also
References
- Voet and Voet. Biochemistry, Third Edition (2004). ISBN 0-471-19350-X. Wiley International Edition.
- Watson, Baker, Bell, Gann, Levine, Losick. Molecular Biology of the Gene, Fifth Edition (2003). ISBN 0-8053-4635-X. Pearson/Benjamin Cummings Publishing.
- Weem, Minka Peeters. International Baccalaureate, Biology, Second Edition (2001). IBID Press, Box 9, Camberwell, 3124, Australia.
- Russell, P. J. 2002. iGenetics. Benjamin Cummings, San Francisco.
- Snyder and Champness. Molecular Genetics of Bacteria, Second Edition (2003). ISBN 1-55581-204-X. ASM Press.
- Bell and Dutta. 2002. Annu. Rev. Biochem 71:333–74.
- Barry, E. R., & Bell, S. D. (2006). DNA replication in the archaea. Microbiology and molecular biology reviews : MMBR, 70(4), 876-887.
- Kelman, L. M., & Kelman, Z. (2003). Archaea: An archetype for replication initiation studies? Molecular microbiology, 48(3), 605-615.
- ^ Berg J, Tymoczko JL, Stryer L (2006). Biochemistry (6th ed. ed.). San Francisco: W. H. Freeman. ISBN 0716787245.
{{cite book}}:|edition=has extra text (help)CS1 maint: multiple names: authors list (link) - ^ DnaA protein binding to individual DnaA boxes in the Escherichia coli replication origin, oriC. C Weigel, A Schmidt, B Rückert, R Lurz, and W Messer
External links
- DNA Workshop
- WEHI-TV - DNA Replication video- Detailed DNA replication animation from different angles with description below.
- Breakfast of Champions Does Replication- creative primer on the process from the Science Creative Quarterly
- Template:McGrawHillAnimation
- Basic Polymerase Chain Reaction Protocol
- Animated Biology
- DNA makes DNA (Flash Animation)
- DNA replication info pageby George Kakaris, Biologist MSc in Applied Genetics and Biotechnology
- Reference website on eukaryotic DNA replication
