Osilodrostat: Difference between revisions
→External links: fix cat |
add EU approval |
||
| Line 18: | Line 18: | ||
| MedlinePlus = |
| MedlinePlus = |
||
| licence_CA = <!-- Health Canada may use generic or brand name (generic name preferred) --> |
| licence_CA = <!-- Health Canada may use generic or brand name (generic name preferred) --> |
||
| licence_EU = |
| licence_EU = Yes |
||
| DailyMedID = Osilodrostat |
| DailyMedID = Osilodrostat |
||
| licence_US = Isturisa |
| licence_US = Isturisa |
||
| Line 52: | Line 52: | ||
| legal_UN = <!-- N I, II, III, IV / P I, II, III, IV--> |
| legal_UN = <!-- N I, II, III, IV / P I, II, III, IV--> |
||
| legal_UN_comment = |
| legal_UN_comment = |
||
| legal_status = |
| legal_status = Rx-only |
||
<!-- Pharmacokinetic data --> |
<!-- Pharmacokinetic data --> |
||
| Line 115: | Line 115: | ||
[[Hypocortisolism]] (low cortisol levels), [[QTc prolongation]] (a heart rhythm condition) and elevations in [[adrenal hormone]] [[Precursor (chemistry)|precursor]]s (inactive substance converted into a hormone) and [[androgen]]s (hormone that regulates male characteristics) may also occur in people taking osilodrostat.<ref name="FDA PR" /> |
[[Hypocortisolism]] (low cortisol levels), [[QTc prolongation]] (a heart rhythm condition) and elevations in [[adrenal hormone]] [[Precursor (chemistry)|precursor]]s (inactive substance converted into a hormone) and [[androgen]]s (hormone that regulates male characteristics) may also occur in people taking osilodrostat.<ref name="FDA PR" /> |
||
Osilodrostat was approved for medical use in the European Union in January 2020,<ref name="Isturisa EPAR">{{cite web | title=Isturisa EPAR | website=[[European Medicines Agency]] | date=12 November 2019 | url=https://www.ema.europa.eu/en/medicines/human/EPAR/isturisa | access-date=7 March 2020}}</ref> and for medical use in the United States in March 2020.<ref name="FDA PR" /> |
|||
Osilodrostat is an [[oral administration|orally active]], [[nonsteroidal]] [[corticosteroid]] [[steroidogenesis inhibitor|biosynthesis inhibitor]] which was developed by [[Novartis]] for the treatment of [[Cushing's syndrome]] and [[pituitary ACTH hypersecretion|pituitary {{abbr|ACTH|adrenocorticotropic hormone}} hypersecretion]] (a specific subtype of Cushing's syndrome).<ref name="pmid27600150">{{cite journal | vauthors = Fleseriu M, Castinetti F | title = Updates on the role of adrenal steroidogenesis inhibitors in Cushing's syndrome: a focus on novel therapies | journal = Pituitary | volume = 19 | issue = 6 | pages = 643–653 | year = 2016 | pmid = 27600150 | pmc = 5080363 | doi = 10.1007/s11102-016-0742-1 | url = }}</ref> It specifically acts as a [[potency (pharmacology)|potent]] and [[binding selectivity|selective]] [[enzyme inhibitor|inhibitor]] of [[aldosterone synthase]] (CYP11B2) and at higher dosages of [[11β-hydroxylase]] (CYP11B1).<ref name="pmid27600150" /> The drug was also under development for the treatment of [[heart failure]], [[hypertension]], and [[solid tumor]]s, but development was discontinued for these indications.<ref name="AdisInsight">http://adisinsight.springer.com/drugs/800026342</ref> |
Osilodrostat is an [[oral administration|orally active]], [[nonsteroidal]] [[corticosteroid]] [[steroidogenesis inhibitor|biosynthesis inhibitor]] which was developed by [[Novartis]] for the treatment of [[Cushing's syndrome]] and [[pituitary ACTH hypersecretion|pituitary {{abbr|ACTH|adrenocorticotropic hormone}} hypersecretion]] (a specific subtype of Cushing's syndrome).<ref name="pmid27600150">{{cite journal | vauthors = Fleseriu M, Castinetti F | title = Updates on the role of adrenal steroidogenesis inhibitors in Cushing's syndrome: a focus on novel therapies | journal = Pituitary | volume = 19 | issue = 6 | pages = 643–653 | year = 2016 | pmid = 27600150 | pmc = 5080363 | doi = 10.1007/s11102-016-0742-1 | url = }}</ref> It specifically acts as a [[potency (pharmacology)|potent]] and [[binding selectivity|selective]] [[enzyme inhibitor|inhibitor]] of [[aldosterone synthase]] (CYP11B2) and at higher dosages of [[11β-hydroxylase]] (CYP11B1).<ref name="pmid27600150" /> The drug was also under development for the treatment of [[heart failure]], [[hypertension]], and [[solid tumor]]s, but development was discontinued for these indications.<ref name="AdisInsight">http://adisinsight.springer.com/drugs/800026342</ref> |
||
Revision as of 07:17, 7 March 2020
 | |
| Clinical data | |
|---|---|
| Trade names | Isturisa |
| Other names | LCI-699 |
| License data | |
| Routes of administration | By mouth |
| Drug class | Steroidogenesis inhibitor |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
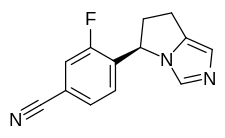
| Formula | C13H10FN3 |
| Molar mass | 227.242 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Osilodrostat, sold under the brand name Isturisa, a medication for the treatment for adults with Cushing's disease who either cannot undergo pituitary gland surgery or have undergone the surgery but still have the disease.[1] It is taken by mouth.[1]
The most common side effects are adrenal insufficiency, headache, vomiting, nausea, fatigue, and edema (swelling caused by fluid retention).[1]
Hypocortisolism (low cortisol levels), QTc prolongation (a heart rhythm condition) and elevations in adrenal hormone precursors (inactive substance converted into a hormone) and androgens (hormone that regulates male characteristics) may also occur in people taking osilodrostat.[1]
Osilodrostat was approved for medical use in the European Union in January 2020,[2] and for medical use in the United States in March 2020.[1]
Osilodrostat is an orally active, nonsteroidal corticosteroid biosynthesis inhibitor which was developed by Novartis for the treatment of Cushing's syndrome and pituitary ACTH hypersecretion (a specific subtype of Cushing's syndrome).[3] It specifically acts as a potent and selective inhibitor of aldosterone synthase (CYP11B2) and at higher dosages of 11β-hydroxylase (CYP11B1).[3] The drug was also under development for the treatment of heart failure, hypertension, and solid tumors, but development was discontinued for these indications.[4]
History
Osilodrostat's safety and effectiveness for treating Cushing's disease among adults was evaluated in a study of 137 adult subjects (about three-quarters women) with a mean age of 41 years.[1] The majority of subjects either had undergone pituitary surgery that did not cure Cushing's disease or were not surgical candidates.[1] In the 24-week, single-arm, open-label period, all subjects received a starting dose of 2 milligrams (mg) of osilodrostat twice a day that could be increased every two weeks up to 30 mg twice a day.[1] At the end of this 24-week period, about half of subjects had cortisol levels within normal limits.[1] After this point, 71 subjects who did not need further dose increases and tolerated the drug for the last 12 weeks entered an eight-week, double-blind, randomized withdrawal study where they either received osilodrostat or a placebo (inactive treatment).[1] At the end of this withdrawal period, 86% of subjects receiving osilodrostat maintained cortisol levels within normal limits compared to 30% of subjects taking the placebo.[1]
The U.S. Food and Drug Administration (FDA) granted osilodrostat an orphan drug designation and granted the approval of Isturisa to Novartis.[1]
See also
References
- ^ a b c d e f g h i j k l "FDA Approves New Treatment for Adults with Cushing's Disease". U.S. Food and Drug Administration (FDA) (Press release). 6 March 2020. Retrieved 6 March 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ "Isturisa EPAR". European Medicines Agency. 12 November 2019. Retrieved 7 March 2020.
- ^ a b Fleseriu M, Castinetti F (2016). "Updates on the role of adrenal steroidogenesis inhibitors in Cushing's syndrome: a focus on novel therapies". Pituitary. 19 (6): 643–653. doi:10.1007/s11102-016-0742-1. PMC 5080363. PMID 27600150.
- ^ http://adisinsight.springer.com/drugs/800026342
Further reading
- Turcu A, Smith JM, Auchus R, et al. (October 2014). "Adrenal androgens and androgen precursors-definition, synthesis, regulation and physiologic actions". Compr Physiol. 4 (4): 1369–81. doi:10.1002/cphy.c140006. PMC 4437668. PMID 25428847. NIHMSID: NIHMS689229.
External links
- "Osilodrostat". Drug Information Portal. U.S. National Library of Medicine.
- Clinical trial number NCT02697734 for "Efficacy and Safety Evaluation of Osilodrostat in Cushing's Disease (LINC-4)" at ClinicalTrials.gov
