Allyl rearrangement
| Mesomeric boundary structures |
|---|
 Allyl radical |
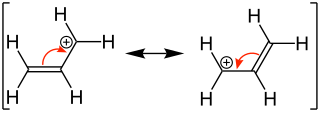 Allyl carbenium ion |
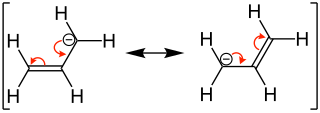 Allyl ion |
The allyl rearrangement in describing organic chemistry the rearrangement of a double bond to mesomeriestabilisierte allyl to form particles.
If there is a substituent on a carbon atom which is in the allyl position , a particularly reactive particle is present. Due to the high reactivity,
- Allyl radicals
- Allyl carbenium ions
- and allyl carbanions
Form a double bond well in the immediate vicinity of the system. The reason lies in the delocalization of the electrons of these formed particles, so that two equivalent mesomeric limit formulas can be described. Particles from which several limit formulas can be drawn are considered to be particularly stable and can be easily formed.
Energetic consideration
The stabilization of the allyl system can also be described with molecular orbitals . Is z. If, for example, the 2-propenyl system is considered, each of the three carbon atoms is sp 2 -hybridized and carries a p orbital perpendicular to the molecular plane. The three p orbitals can be combined in such a way that three orbitals arise. Of the three new molecular orbitals, one is binding and has no nodal plane ( 1 ), one is non-binding and has a nodal plane ( 2 ) and one is antibonding with two nodal planes ( 3 ). The non-binding orbital is energetically on the level of a non-interacting p-orbital. The orbitals can be filled with the appropriate number of electrons, whereby the allyl carbenium ion has only one occupied orbital with two electrons and the allyl radical and allyl carbanion occupies another orbital with one or two electrons. In all cases the total energy is lower than that of three non-interacting p orbitals, which explains the great stability of the particles.
Example reactions
Radical substitution
Allyl compounds can react in a radical substitution reaction, whereby the allyl shift explains the variety of products. In the example of propene, a low CH bond strength (364 kJ / mol) can be measured. This primary CH bond is thus even weaker than a tertiary CH bond, so that the allyl radical is particularly stabilized and can be easily formed with the help of light energy. As soon as further radicals, for example bromine radicals, are present, two isomeric products can be formed.
Acid-base reaction
Allyl carbanions are usually formed by removing an atom or another leaving group. The splitting off of a proton with strong bases such as NaNH 2 or C 4 H 9 Li is particularly popular .
For example, propene, with an acidity of pK a = 40, is more acidic than propane (pK a = 50). The formation of the allyl anion is thus favored, whereby the hydrogen atom can be split off.
Nucleophilic substitution
Allyl compounds very easily enter into nucleophilic substitution reactions , whereby the pronounced rearrangement tendency becomes clear. In the example of 3-chloropropene, there is a chlorine atom on a carbon atom which is in the allyl position. In contrast to saturated primary haloalkanes, 3-chloropropene dissociates relatively quickly under S N 1 conditions and undergoes rapid substitution via a cationic intermediate. The carbenium ion formed from 3-chloropropene appears to be more stable than other primary carbenium ions, which are not so easily formed. When measured precisely, the stability corresponds to that of a secondary carbenium ion.
The shift of the double bond often observed in the reaction of allyl halides with nucleophiles , such as bromide ions, is based on the intermediate formation of a mesomeric-stabilized carbenium ion, which has two potential binding sites for the substituent entering. A total of two isomeric compounds can be formed.
Individual evidence
- ↑ a b c d e f g h K. Peter C. Vollhardt, Neil E. Schore: Organic chemistry . Ed .: Holger Butenschön. 4th edition. Wiley-VCH, Weinheim 2007, ISBN 3-527-31380-X , pp. 682-685 .
- ^ A b Jonathan Clayden, Nick Greeves, Stuart Warren: Organic Chemistry . 2nd Edition. Springer, Berlin 2013, ISBN 978-3-642-34715-3 , pp. 167-169 .
- ^ A b Hans Peter Latscha, Uli Kazmaier, Helmut Alfons Klein: Organic chemistry . 6th edition. Springer Spectrum, Heidelberg 2013, ISBN 978-3-642-36592-8 , p. 331 .
- ^ A b Wolfgang Uhl, Apostolos Kyriatsoulis: Name and catchword reactions in organic chemistry . Vieweg, Braunschweig 1984, ISBN 978-3-528-03581-5 , p. 191-192 .


