Aston Greenburg rearrangement
The Aston-Greenburg rearrangement is a name reaction in organic chemistry . The chemists JG Aston and RB Greenburg first reported this reaction in 1940. It describes the rearrangement of an α- haloketone into an ester with a tertiary α- carbon atom.
The rearrangement takes place via the intramolecular migration of an alkyl or aryl group .
Overview reaction
The reaction can be carried out with sodium methoxide in diethyl ether and is stereospecific. The overview reaction is shown here in a simplified manner using the example of the reaction of 3-bromo-3-methyl-2-butanone to methyl trimethylacetate, with the rearranged methyl group being marked in blue :
Reaction mechanism
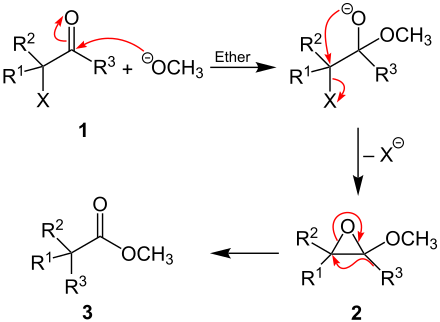
The exact mechanism of the reaction is not known. A proposed mechanism, which is similar to the Favorskii rearrangement , follows . As an example, the reaction with sodium methoxide is carried out on an unspecified α- halo ketone (at least R 1 and / or R 2 and in each case R 3 is an organic radical and X is a halogen, where R 3 in the literature as alkyl or Aryl group is described). In the first step, the alkoxylate attacks the positively polarized carbon atom of the keto group of the α- halo ketone ( 1 ). The halogen is then split off as an anion with the formation of an epoxide ( 2 ). The formation of the ester ( 3 ) finally takes place via the intramolecular migration of the alkyl or aryl group (R 3 ) and with the formation of the carbonyl group from the epoxide:
Competitive reaction
In diethyl ether, the reaction proceeds with a predominant yield of the ester with a tertiary α carbon atom. Adding the alcohol, for example methanol when using sodium methoxide , can lead to an addition of an alcohol molecule to the epoxide, which means that the rearrangement of the alkyl or aryl group does not occur. A hydroxyacetal is formed, shown as a reaction product using the example of the reaction of 3-bromo-3-methyl-2-butanone and sodium methoxide. When the reaction is carried out in absolute alcohol, the hydroxyacetal is predominantly formed with a low yield of the ester with a tertiary α carbon atom.
Atomic economy
The atom economy of this reaction can in principle be assessed as efficient, since relatively few by-products are formed in the context of rearrangement reactions. Due to the elimination of the halogen and the resulting sodium salt (when using sodium alcoholate), however, small amounts of waste products must be taken into account. In addition, the possible formation of hydroxyacetal reduces the efficiency of this reaction. In the context of a Claisen rearrangement, for example, a hydroxyacetal that is formed could be reused in an E -stereoselective reaction by reacting with an allyl alcohol , which would improve the economy of the reaction.
Web links
Individual evidence
- ↑ a b c d e f Daniel Zerong Wang: Comprehensive Organic Name Reactions and Reagents . tape 1 . John Wiley & Sons, Inc., John Wiley & Sons, Inc., Hoboken, New Jersey 2009, ISBN 978-0-471-70450-8 , pp. 111-113 .
- ↑ a b c d e f g h i J. G. Aston, RB Greenburg: alpha-Bromo Secondary Alkyl Ketones. I. Reaction with Sodium Alcoholates. A New Synthesis of Tertiary Acids by Rearrangement. In: Journal of the American Chemical Society. Volume 62, No. 10, 1940, pp. 2590-2595, DOI: 10.1021 / ja01867a003 .
- ^ CR Engel, G. Just: Steroids and Related Products. I. The Synthesis of 17 α-Methyldeoxycorticosterone. In: Journal of the American Chemical Society. Volume 76, 1954, pp. 4909-4914, DOI: 10.1021 / ja01648a044 .
- ↑ Takayanagi Hisao, Morinaka Yasuhiro: Highly Stereoselective Synthesis of Trisubstituted γ, δ-Unsaturated Acid and Aldehyde via Ketal Claisen Rearrangement. In: Chemistry Letters. Volume 24, No. 7, 1995, pp. 565-566, DOI: 10.1246 / cl.1995.565 .