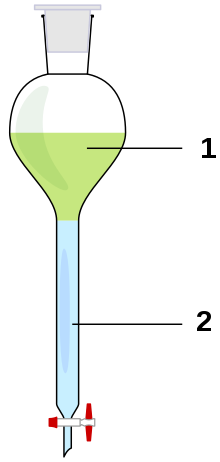
Shake out
In chemistry, shaking is a physical separation process in which a dissolved substance is extracted using another solvent . In technical terms, liquid-liquid extraction is used.
For this purpose, the solution with the substance to be extracted is filled into a separating funnel and a (defined) amount of a solvent is added to absorb the substance (or a reaction product). The added solvent should not (or only insignificantly) mix with the other solvent, so that a mixture results that consists of two solvent phases . The separating funnel is shaken by hand or a machine for a while - hence the name of the method. After a waiting phase, one of the liquid phases can be drained and thus a separation can be achieved. With Nernst's law of distribution one can describe the concentration distribution between the phases.