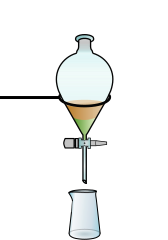
Separating funnel
A separating funnel (also known as a shaking funnel ) is a glass container that is used in the chemical laboratory to separate immiscible liquids.
The device has a standard ground joint at the top , which can be closed with a stopper, and a tap at the bottom. If two immiscible liquids (two phases ) are poured in (e.g. water and oil), the liquid with the greater density collects at the bottom (i.e. water in the case of water and oil) and can be drained into another vessel via the tap become. The conical shape of the separating funnel, which becomes narrower towards the bottom, facilitates an exact separation, i.e. closing the tap, just before the lighter phase begins to run out.
use
In organic-chemical laboratory technology, the separating funnel is used to extract substances from a solution : A solvent is added which dissolves the substance to be extracted better, but cannot be mixed with the previous solution. After repeated shaking, phase separation, draining and adding fresh solvent, the substance to be extracted is enriched in the new solvent. This behavior is described for the solute using Nernst's distribution theorem. This liquid-liquid extraction , called “ shaking out ” in laboratory jargon, is a material separation process and a cleaning method .
Web links
Individual evidence
- ↑ Organikum . 23rd edition. Wiley-VCH Verlag GmbH, 2009, ISBN 978-3-527-32292-3 , pp. 60-61.
- ↑ Walter Wittenberger: Chemical laboratory technology: an auxiliary book f. Laboratory technicians u. College student . 7., completely reworked. Edition. Springer, Vienna / New York 1973, ISBN 3-211-81116-8 .
- ↑ Michael Wächter: Chemielabor. Introduction to laboratory practice . 1st edition. Wiley-VCH, Weinheim 2011, ISBN 978-3-527-32996-0 .