Benary reaction
The Bénary reaction is a name reaction in organic chemistry that was first described in 1909 by Erich Bénary (1881–1941) . Here, ketones of enamines are converted with Grignard compounds . There arise β-unsaturated α, ketones, aldehydes or esters .
Overview reaction
An enaminoketone reacts with a Grignard compound to form an α, β-unsaturated ketone:
The blue radical R is an organic alkyl radical , for example an ethyl radical .
Reaction mechanism
Zerong Wang suggests two mechanisms. Both are shown below, with the 1,4-addition-elimination mechanism believed to be more likely.
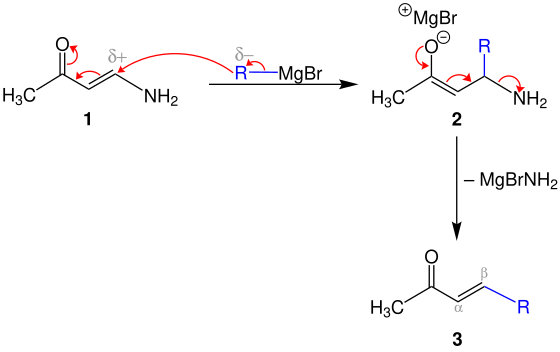
1,4-addition-elimination mechanism
In the first step, the blue alkyl the Grignard reagent is applied to the positively polarized carbon atom of the enaminoketone 1 added , whereby the magnesium - enolate 2 is formed.
The elimination of MgBrNH 2 produces the α, β-unsaturated ketone 3 .
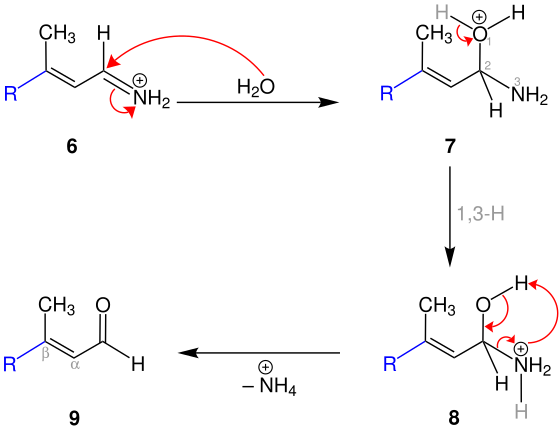
1,2-addition mechanism
In this case the alkyl radical of the Grignard compound is added to the positively polarized carbonyl carbon atom of 1 . The resulting magnesium enolate 4 is then treated with water. The protonation of the hydroxy group of the resulting amino enolate 5 results in the formation of the cation 6 .
Also, 6 is protonated by water, whereby the oxonium ion 7 is formed.
The subsequent 1,3-hydrogen shift creates the cation 8 . Finally, an ammonium cation is eliminated. This creates an α, β-unsaturated aldehyde 9 .
literature
- Z. Wang: Comprehensive Organic Name Reactions and Reagents , Vol 1. John Wiley & Sons, Hoboken, New Jersey 2009 , pp. 311-314, ISBN 978-0-471-70450-8 .
Individual evidence
- ↑ Erich Bénary: About the acylation of the β-amino-crotonic acid ester and related compounds , reports of the German chemical society 1909 , 42 (3) , 3912–3925, doi : 10.1002 / cber.190904203147 .
- ↑ Erich Bénary: About a mode of formation of unsaturated ketones from substituted amino-methyl ketones , reports of the German chemical society 1931 , 64 (9) , 2543-2545, doi : 10.1002 / cber.19270600539 .
- ↑ Erich Bénary: On the effect of ammonia and amines on some aliphatic and aromatic oxymethylene ketones , reports of the German chemical society 1930 , 63 (6) , 1573–1577, doi : 10.1002 / cber.19300630641 .
- ↑ Ferdinand Näf, René Decorzant: A Stereospecific Synthesis of (E, Z) -α, β-γ, δ-Diunsaturated Aldehydes, Ketones, and Esters Using the Benary Reaction , Helvetica Chimica Acta 1974 , 57 (5) , 1309-1317 , doi : 10.1002 / hlca.19740570507 .