Babler oxidation
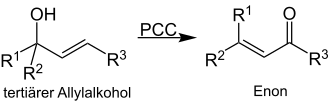
The Babler oxidation is a name reaction in organic chemistry in which a tertiary allyl alcohol is oxidized with pyridinium chlorochromate (PCC). This creates an enone :
This reaction creates a conjugated aldehyde or a conjugated ketone . The reaction is carried out in a dried solvent (e.g. dichloromethane or chloroform ).
Reaction mechanism
At the beginning a chromate tester 1 is created . Another chromate ester 2 is formed through a [3,3] sigmatropic rearrangement . Then 2 is oxidized to the conjugated enone 3 :
Instead of PCC, TEMPO can also be used as an oxidizing agent.
See also
Individual evidence
- ↑ JH Babler, MJ Coghlan: A Facile Method for the Bishomologation of Ketones to α, β-Unsaturated Aldehydes: Application to the Synthesis of the Cyclohexanoid Components of the Boll Weevil Sex Attractant , Synth. Commun. 6 (1976) pp. 469-474. {Doi = 10.1080 / 00397917608082626}
- ↑ M. Shibuya, M. Tomizawa, Y. Iwabuchi: Oxidative Rearrangement of Tertiary Allylic Alcohols Employing Oxoammonium Salts, J. Org. Chem. 73 (2008), pp. 4750-4752, {doi = 10.1021 / jo800634r}.