Baeyer-Drewson reaction
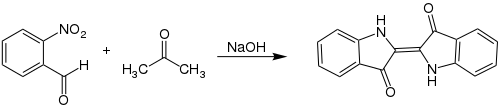
The Baeyer-Drewson reaction , also called Baeyer-Drewson Indigo synthesis , is a name reaction in organic chemistry , which was first introduced in 1882 by Adolf Baeyer (1835-1917) and Viggo Drewson and named after them. This reaction is a synthesis of indigo from 2-nitrobenzaldehyde and acetone .
Overview reaction
In the Baeyer-Drewson reaction, indigo is produced by a condensation reaction of 2-nitrobenzaldehyde and acetone in the presence of dilute sodium hydroxide solution .
Reaction mechanism
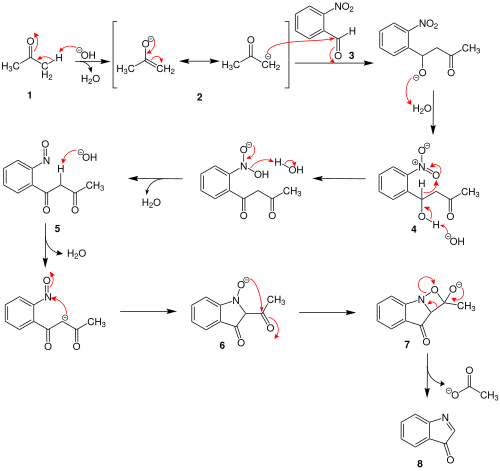
In the proposed reaction mechanism, the acetone ( 1 ) is deprotonated using the sodium hydroxide solution in the first step . It forms an enolate ion 2 , which attacks 2-nitrobenzaldehyde ( 3 ) nucleophilically . After protonation, intermediate 4 is formed . This intermediate product is first deprotonated on the hydroxyl group and then protonated on the nitro group, whereby water is split off. The intermediate product 5 is formed . This is followed by the deprotonation of an acidic hydrogen atom. The anion 6 is formed after ring closure . A further ring closure and the elimination of an acetate anion ( 7 ) results in intermediate product 8 .
This intermediate product 8 then reacts first with a water molecule and then with a hydroxide ion with elimination of water. The result is the ion 9 , which attacks another equivalent of the intermediate product 8 . After two further reaction steps, releasing a hydroxide ion, indigo ( 10 ) is formed.
modification
In 1880–1882, Adolf Baeyer discovered two synthetic routes for the production of indigo. The second synthetic route was via cinnamic acid . However, this procedure could not prevail.
application
The greatest application of the Baeyer-Drewson reaction is in the synthesis of indigo derivatives. It can also be used to test for the presence of methyl ketones .
See also
Individual evidence
- ^ Adolf Baeyer, Viggo Drewsen: Representation of indig blue from orthonitrobenzaldehyde . In: Reports of the German Chemical Society . tape 15 , no. 2 , 1882, p. 2856-2864 , doi : 10.1002 / cber.188201502274 .
- ↑ a b c d Wang, Zerong (Daniel Zerong): Comprehensive organic name reactions and reagents . John Wiley, Hoboken, NJ 2009, ISBN 978-0-471-70450-8 , pp. 136-139 .
- ↑ Namboothiri, I. (Irishi): Organic syntheses based on name reactions: a practical guide to 750 transformations . 3rd ed.Elsevier, Amsterdam 2012, ISBN 978-0-08-096631-1 , pp. 19 .
- ↑ Helmut Schmidt: Indigo - 100 years of industrial synthesis . In: Chemistry in Our Time . tape 31 , no. 3 , 1997, p. 121–128 , doi : 10.1002 / ciuz.19970310304 .