Bimolecular fluorescence complementation
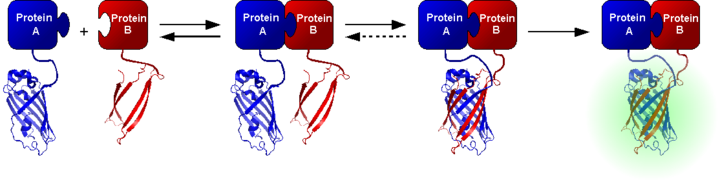
The bimolecular fluorescence complementation (engl. Bimolecular fluorescence complementation , BiFC ) is a method of molecular biology for the detection of protein-protein interactions . The method is based on the complementation of two non- fluorescent fragments of a fluorescent protein, such as green fluorescent protein (GFP). By assembling the two fragments, an intact, fluorescent complex is created. The process of bimolecular fluorescence complementation was largely developed by Tom Kerppola and colleagues.
principle
The principle of bimolecular fluorescence complementation is related to enzyme fragment complementation methods and two-hybrid systems . To examine protein-protein interactions, two different fragments of a fluorescent protein are attached to the two proteins to be examined with the aid of molecular biological methods. Fragments of the yellow fluorescent protein (YFP), which consist of the first (N-terminal) 155 amino acids or the last (C-terminal) 83 amino acids, are usually used, but different fragments or fragments of other fluorescent proteins can also be used Both fragments as a result of an interaction of the proteins to be examined and coupled to the fragments in the immediate vicinity, can be aggregated and complemented to form an intact total crude protein, which is capable of fluorescence after maturation . The fluorescence can be detected and quantified with the aid of fluorescence microscopy or fluorescence spectroscopy .
The extent of complementation is largely dependent on physical contact between the complementary fragments and thus on an interaction of the test proteins coupled to them. Since BiFC complexes are stable once they have formed, short interactions between the proteins investigated in living cells can also be detected. In contrast to the FRET and BRET methods based on resonance energy transfer , the bimolecular fluorescence complementation, due to the slow maturation of fluorescent proteins, does not allow a time-resolved investigation of molecular interactions. Limiting the use of bimolecular fluorescence complementation is the position of the test protein-fluorescent protein fragment coupling, which can be decisive for complementation, and the rate of self-complementation of the fluorescent protein fragments, which can lead to false positive interpretations.
Variations
Newer variants are based on a complementation of fragments of different colored fluorescent proteins, whereby several protein-protein interactions can be detected at the same time (multicolor fluorescence complementation). By combining the bimolecular fluorescence complementation with FRET or BRET, tri- or oligomeric protein complexes can also be detected.
Individual evidence
- ↑ Hu CD, Chinenov Y, Kerppola TK: Visualization of interactions among bZIP and Rel family proteins in living cells using bimolecular fluorescence complementation . In: Molecular cell . 9, No. 4, April 2002, pp. 789-98. PMID 11983170 .
- ↑ a b Hu CD, Kerppola TK: Simultaneous visualization of multiple protein interactions in living cells using multicolor fluorescence complementation analysis . In: Nature biotechnology . 21, No. 5, May 2003, pp. 539-45. doi : 10.1038 / nbt816 . PMID 12692560 . PMC 1820765 (free full text).
- ↑ Kerppola TK: Bimolecular fluorescence complementation (BiFC) analysis as a probe of protein interactions in living cells . In: Annual review of biophysics . 37, 2008, pp. 465-87. doi : 10.1146 / annurev.biophys.37.032807.125842 . PMID 18573091 .
- ↑ Shyu YJ, Suarez CD, Hu CD: Visualization of ternary complexes in living cells by using a BiFC-based FRET assay . In: Nature Protocols . 3, No. 11, 2008, pp. 1693-1702. doi : 10.1038 / nprot.2008.157 . PMID 18846096 .