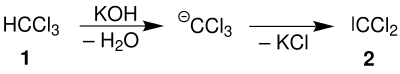Ciamician-Dennstedt rearrangement
The Ciamician-Dennstedt rearrangement is a name reaction in organic chemistry and was first published in 1881 by the Italian chemist Giacomo Luigi Ciamician and his German colleague Max Dennstedt . The reaction describes the conversion of pyrrole into halogen-containing pyridines .
Overview reaction
Pyrrole reacts with a trihalomethane in an alkaline solution to form a halopyridine.
Reaction mechanism
The mechanism is described in the literature and visualized using the example reaction of chloroform with pyrrole in potassium hydroxide :
Chloroform ( 1 ) reacts in an alkaline solution (potassium hydroxide), splitting off water and potassium chloride, to form dichlorocarbene ( 2 ):
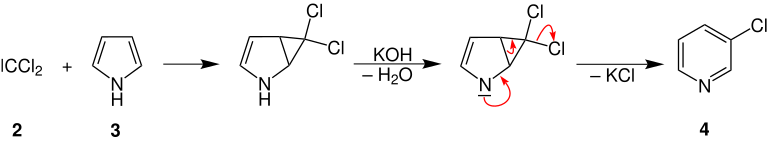
The dichlorocarbene ( 2 ) then reacts with pyrrole ( 3 ) and another base (potassium hydroxide), with elimination of water and potassium chloride, to form 3-chloropyridine ( 4 ):
application
Analogous to the Ciamician-Dennstedt rearrangement, methyl-substituted indene reacts with dichlorocarbene with ring expansion to form 2-chloro-3-methylnaphthalene:
Individual evidence
- ↑ GL Ciamician M. Dennstedt (1881), On the action of chloroform on the potassium compound pyrrole, Ber. German Chem. Ges. A / B, 14: 1153-1163, doi: 10.1002 / cber.188101401240 .
- ↑ Elliot R. Alexander, Aaron B. Herrick, and Thomas M. Roder: The Formation of 3-Substituted Pyridines from Pyrrole In: J. Am. Chem. Soc. 72 (6), 1950, pp. 2760-2761, doi: 10.1021 / ja01162a501 .
- ↑ a b Z. Wang: Comprehensive organic name reactions and reagents Volume 1 . John Wiley, Hoboken (NJ) 2009, ISBN 978-0-470-28662-3 , pp. 646-648 .
- ^ William E. Parham and Charles D. Wright: Formation of Naphthalenes from Indenes. IV.1 The Effect of Substitution at the Ethylenic Double Bond In: J. Org. Chem. 22 (11), 1957, pp. 1473-1477, doi: 10.1021 / jo01362a041 .