Gomberg radical reaction
The Gomberg radical reaction is a name reaction in organic chemistry . The radical reaction was named after the American chemist Moses Gomberg (1866–1947). In 1900 Gomberg described the first permanent free radical (more precisely triphenylmethyl radical , also known as the Gomberg radical). This is the first synthesis of an organic radical, creating a new field in chemistry.
Overview reaction
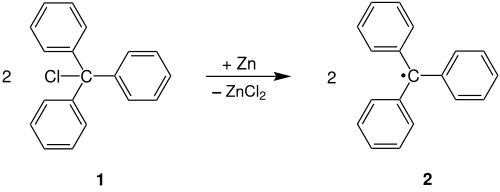
The Gomberg radical reaction is used to produce the triphenylmethyl radical 2 by abstraction of a chlorine radical from triphenylchloromethane 1 under the influence of zinc. Instead of zinc, other metals such as. B. silver can be used.
Reaction mechanism
During the reaction there is a one-electron transfer from the metal (in the example silver) to the triphenylchloromethane 1 . A triphenylmethyl radical 2 is formed by this reduction .
If two such triphenylmethyl radicals combine, a dimerization product is obtained.
Dimerization product
In 1900 Gomberg assigned the structural formula 3 to the dimerization product of the triphenylmethyl radical 2 .
In 1970 it was demonstrated by means of NMR spectroscopy that the dimerization product is not hexaphenylethane 3 , but an asymmetrical dimer : 3-diphenylmethylene-6-triphenylmethyl-cyclohexa-1,4-diene 4 :
Individual evidence
- ^ Louis Fieser, Mary Fieser: Organic Chemistry , 2nd Edition, Verlag Chemie, Weinheim 1972, ISBN 3-527-25075-1 , pp. 409-413.
- ^ Thomas T. Tidwell: The First Century of Ketenes (1905–2005): The Birth of a Versatile Family of Reactive Intermediates , Angewandte Chemie, Int. Edition , Volume 44, 2005, pp. 5778-5785; doi: 10.1002 / anie.200500098 .
- ↑ JM McBride, Tetrahedron , Volume 30, 1974, pp. 2009-2022.
- ↑ M. Gomberg, Ber. Deutsche Chem. Ges. , Volume 33, 1900, pp. 3150-3163.
- ↑ M. Gomberg, J. Am. Chem. Soc. , Volume 22, 1900, pp. 757-771.
- ^ A b Jonathan Clayden, Nich Greeves, Stuart Warren: Organische Chemie, 2nd edition, 2013, Springer Spectrum, ISBN 978-3-642-34715-3 , p. 1066.
- ^ Siegfried Hauptmann : Organic chemistry . 2nd edition, VEB Deutscher Verlag für Grundstoffindustrie, Leipzig 1985, ISBN 3-342-00280-8 , p. 281.