Bomb calorimeter
A bomb calorimeter (also known as Berthelot's bomb) is used to determine the calorific value of a substance under an oxygen atmosphere and high pressure.
Structure and functionality
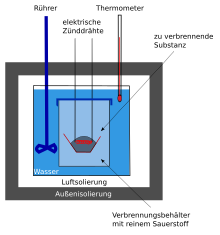
Bomb calorimeters consist of a steel container filled with temperature-controlled water (typically 2,000 g), which can be assumed to be adiabatic and in which, in addition to the actual bomb made of high-strength chrome steel, there is a stirrer and a thermometer. In the bomb, which typically has an internal volume of 340 cm 3 , there is a suspension for a crucible ; the suspension also serves as an electrical conductor for the ignition wire, which lies in the substance to be burned. It is then ignited either by an electric arc or by the heat of the ignition wire through which current flows. The combustion takes place at an oxygen pressure of around 20–30 bar . A solid (0.5–1.5 g) is usually burned in the form of a so-called pellet . The pellet shape of the pellet prevents particles from being thrown out of the crucible during the explosive combustion and from cooling below the flash point on the cold bomb walls.
During the combustion, the temperature in the bomb increases, this heat is then given off to the water and the temperature change in the water is then precisely measured with a thermometer. By measuring the temperature rise of the bomb calorimeter, conclusions can be drawn about the calorific value .
There are several measurement principles. The most common are the adiabatic and isoperibolic procedures.
Thermograms
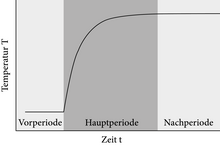
A thermogram shows the temperature profile T (t) recorded during the reaction. It is divided into three sections: the previous period, the main period (also called jump area) and the post-period. In an ideal adiabatic experiment, the system shows a constant temperature in the previous period, which after the start of the reaction approaches the new equilibrium temperature (post-period) exponentially, which then also remains constant. The thermograms of non-ideal adiabatic calorimeters and isoperibolic calorimeters can only approximately reproduce the ideal temperature profile T (t), since the system is often not fully equilibrated before the measurement. As a result, the thermogram still shows a slight temperature drift during the pre- and post-period.
The example graph of the thermogram of a non-ideal adiabatic calorimeter shows that the reaction was started at the end of the previous period at time t = 600 s. The straight line lying perpendicular to the linear regression of the previous and main period results in two areas of equal size F 1 and F 2 . The intersection of the vertical and the main period gives the mean reaction temperature at 63% of the temperature jump.
Calculation of thermodynamic quantities
To calculate thermodynamic quantities from the data obtained, the heat capacity (calorimeter constant) of the bomb calorimeter must first be determined.If the substance is known, this is done using the internal energy of this substance, the mass difference before and after combustion and the temperature difference generated :
With the help of the specific heat capacity of the water , the amount of water used and the value, it is possible to determine the water value of the calorimeter:
If the thermodynamic data of the substance are not known, the heat capacity of the calorimeter must first be determined using a known substance, as shown above. From this, the internal energy of the unknown substance can be determined if the molar mass is known:
The enthalpy of reaction is then calculated using:
Assuming that the gas phase can be assumed to be ideal, it is possible to rewrite the equation as:
is the difference in the amount of substance resulting from the reaction equation. If the gas phase cannot be assumed to be ideal, other equations must be used, e.g. B. the Van der Waals equation .
See also
Individual evidence
- ↑ Spektrum.de: Bomb Calorimeter - Lexicon of Nutrition - Spectrum of Science , accessed on February 12, 2017.
- ↑ Technical University of Munich, measurement technology for bomb calorimeters
- ↑ a b c d Erich Meister: Basic practical course in physical chemistry - theory and experiments . 2nd Edition. vdf Hochschulverlag AG, Zurich 2012, ISBN 978-3-7281-3709-8 , p. 179–186 ( limited preview in Google Book search).
- ↑ Aalen University Determination of the combustion energy with the bomb calorimeter
- ^ University of Jena Molar enthalpy of combustion and formation with the bomb calorimeter
- ↑ Gabriele Cruciani: Short textbook physical chemistry . John Wiley & Sons, 2006, ISBN 3-527-31807-0 , pp. 134 ( limited preview in Google Book search).