Kauffmann olefination
A Kauffmann olefination is a reaction developed by Thomas Kauffmann to convert a ketone or an aldehyde into a methylene group ( methylenation ). The reaction is related to the Tebbe reaction , the Wittig reaction or the Lombardo reagent .
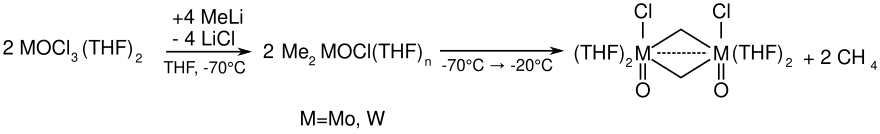
Preparation of the reagent
The reagent for the Kauffmann olefination is produced in situ from various molybdenum or tungsten chlorides by reaction with methyl lithium at low temperatures (−78 ° C).
The olefination reagent is produced when the reaction mixture is heated up. It could be shown on the basis of NMR studies that the reactive species is not a Schrock carbene (such as the Tebbe reagent ).
mechanism
Mechanistic studies have shown that the olefination process is a sequence of cycloaddition and cycloelimination steps.
operation area
The Kauffmann olefination has for a long time received practically no attention in the literature. More recently, however, it has found application in natural product synthesis as a very mild and non-basic olefination reagent. In the course of this work a combination of the olefination step and an olefin metathesis was developed. It is noteworthy that the inorganic reaction products of the Kauffmann olefination do not decompose the metathesis catalyst.
Individual evidence
- ↑ T. Kauffmann, M. Papenberg, R. Wieschollek, J. Sander: "Alkylchromium and Alkylmanganese Reagents, IV. - On the Aldehydes and Cheleselective Alkylation of Organic Carbonyl Compounds with Monoalkylchromium (III) Reagents." in Chem. Ber. 1992 , 125 , 143-148. doi : 10.1002 / cber.19921250126
- ↑ T. Kauffmann, P. Fiegenbaum, M. Papenberg, R. Wieschollek, D. Wingbermühle: "Organomolybdenum and organotungsten; reagents, III. Chemoselective, non-basic carbonylmethylenation; reagents from MoOCl 3 (THF) 2 and MoOCl 4 : Education, thermolability, structure "in Chem. Ber. 1993 , 126 , 79-87. doi : 10.1002 / cber.19931260114
- ↑ T. Kauffmann, J. Braune, P. Fiegenbaum, U. Hansmersmann, C. Neiteler, M. Papenberg, R. Wiescholleck: In Chem. Ber. 1993 , 126 , 89-96.
- ↑ T. Kauffmann: In Angew. Chem. 1997 , 109 , 1312-1329.
- ↑ K. Oesterreich, D. Spitzner: In Tetrahedron 2002 , 58 , 4331-4334.
- ↑ K. Oesterreich, I. Klein, D. Spitzner: In Synlett 2002 , 1712-1714.