Knorr quinoline synthesis
The Knorr quinoline synthesis , named after the German chemist Ludwig Knorr , is a name reaction from the field of organic chemistry and was first described in 1886. The Knorr quinoline synthesis enables the preparation of quinoline from aniline and β-ketoesters .
Overview reaction
With elimination of water and alcohol (in the example: methanol), 2-hydroxyquinoline can be produced by cyclization from aniline and a β-keto ester. The reaction is acid-catalyzed and takes place at high temperatures:
The Knorr quinoline synthesis is similar to the Conrad Limpach quinoline synthesis , with which 4-hydroxyquinoline can be produced at low temperatures.
Reaction mechanism
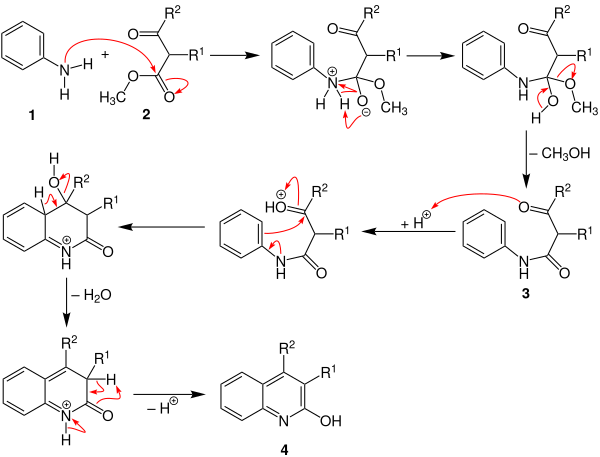
The following reaction mechanism is described in the literature:
First of all, the amino group of the aniline ( 1 ) attacks the β-ketoester 2 . The anilide 3 is formed by proton rearrangement and subsequent elimination of methanol . After the subsequent protonation, a shift in the electrons leads to ring closure. 2-Hydroxyquinoline 4 is finally formed through elimination of water , deprotonation and subsequent tautomerism .
further reading
- Charles R. Hauser and George A. Reynolds: Reactions of β-Keto Esters with Aromatic Amines. Syntheses of 2- and 4-Hydroxyquinoline Derivatives In: Journal of the American Chemical Society . Volume 70, 1948, pp. 2402-2404, doi: 10.1021 / ja01187a025 .
Individual evidence
- ↑ Ludwig Knorr: Synthetic experiments with the acetoacetic ester. In: Justus Liebig's Annals of Chemistry . Volume 236, 1886, pp. 69-115, doi: 10.1002 / jlac.18862360105 .
- ↑ Helmut Krauch, Werner Kunz, Eberhard Nonnenmacher: Reactions of organic chemistry. WILEY ‐ VCH, 2009, ISBN 9783527297139 , doi: 10.1002 / jlac.18862360105 , pp. 400–401.
- ^ Zerong Wang: Comprehensive Organic Name Reactions and Reagents. Wiley, 2009, ISBN 978-0-471-70450-8 , pp. 1638-1641.