LNA (nucleic acid)
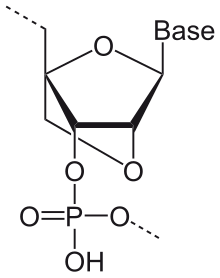
LNA ( English locked nucleic acid , German bridged nucleic acid ) is a xeno nucleic acid and consists of the modified nucleotides . The ribose unit of the RNA building blocks is linked in the LNA with an additional bridge between the 2'-oxygen and 4'-carbon.
This fixes the ribose in the 3'-endo (north) conformation and is structurally more inflexible than the unmodified analogue. This significantly increases the hybridization properties (melting point) and the affinity for base stacking.
LNAs were first published independently by Takeshi Imanishi et al. and Jesper Wengel et al. synthesized.
A Danish biotech company (Exiqon A / S) secured exclusive rights to the LNA technology in 1997.
In contrast to LNA is UNA ( English unlocked nucleic acid , German unbridged nucleic acid).
advantages
The therapeutic use of LNA-based oligonucleotides is an emerging area in biotechnology . The modified building blocks can be used like normal nucleosides in solid-phase synthesis.
Some of the advantages of LNA technology:
- Ideal for the detection of short RNA and DNA targets
- Increase the thermal stability
- Complete resistance to exo- and endonucleases (consequently high stability in vivo and in-vitro applications)
- Increased target specificity. Allele-specific PCR using LNA allows the design of shorter primers without compromising the binding specificity.
- Compatibility with standardized enzyme processes
Individual evidence
- ^ A. Arora, J. Wengel: Thermodynamic, Counterion, and Hydration Effects for the Incorporation of Locked Nucleic Acid Nucleotides into DNA Duplexes. in: Biochemistry. 2006 , 45 (23), pp. 7447-7455; doi : 10.1021 / bi060307w .
- ^ Y. Hari, T. Imanishi: Synthesis of 2'- O , 4'- C -methyleneuridine and -cytidine. Novel bicyclic nucleosides having a fixed C3'- endo sugar puckering. in: Tetrahedron Letters. 1997 , 38 (50), pp. 8735-8738; doi : 10.1016 / S0040-4039 (97) 10322-7 .
- ↑ A. Alexei, J. Wengel: LNA (Locked Nucleic Acids): Synthesis of the adenine, cytosine, guanine, 5-methylcytosine, thymine and uracil bicyclonucleoside monomers, oligomerisation, and unprecedented nucleic acid recognition. in: Tetrahedron Letters. 1998 , 54 (14), pp. 3607-3630; doi : 10.1016 / S0040-4020 (98) 00094-5 .
- ↑ homepage Exiqon
- ↑ Niels Langkjær, Anna Pasternak, Jesper Wengel: UNA (unlocked nucleic acid): A flexible RNA mimic that allows engineering of nucleic acid duplex stability. In: Bioorganic & Medicinal Chemistry . Volume 17, No. 15, 2009, pp. 5420-5425, doi : 10.1016 / j.bmc.2009.06.045 .
- ↑ M. A. Campbell, J. Wengel: Locked vs. unlocked nucleic acids (LNA vs. UNA): contrasting structures work towards common therapeutic goals. In: Chemical Society reviews. Volume 40, No. 12, December 2011, pp. 5680-5689, doi : 10.1039 / c1cs15048k , PMID 21556437 (review).
- ↑ Petersen M, Wengel J: LNA: a versatile tool for therapeutics and genomics . In: Trends Biotechnol. . 21, No. 2, February 2003, pp. 74-81. doi : 10.1016 / S0167-7799 (02) 00038-0 . PMID 12573856 .
- ↑ M. Frieden, HF Hansen, T. Koch: Nucleosides, Nucleotides and Nucleic Acids. 2003 , 22, pp. 1041-1043.
- ↑ Bonetta L: Prime time for real-time PCR . In: Nat. Methods . 2, No. 4, 2005, pp. 305-312. doi : 10.1038 / nmeth0405-305 .