Xenonucleic acid
Xenonucleic acids ( English Xeno nucleic acid , short XNA ) are artificial variants of the natural nucleic acids RNA and DNA and thus part of synthetic biology . XNA is a synthetic polymer that can carry the same information as DNA, but with different molecular components. An essential feature is that the sugar molecule ( ribose or deoxyribose ) is replaced by another sugar (for example threose or a hexose ), a sugar analog (such as ethylene glycol ) or another group. The development of six different of these artificial XNA nucleotides was submitted for publication in December 2011 by Vitor B. Pinheiro and colleagues. Like RNA and DNA, these can form nucleic acid bridging chains, which means that genetic information can be stored and retrieved. The prefix “Xeno” (and thus the “X” in XNA) is derived from the Greek ξένος and means “foreign” or “foreign body”, which refers to the difference in the molecular structure compared to DNA or RNA.
Even if there are no non-standard bases , i.e. H. the genetic information is stored in the four canonical DNA bases , natural DNA polymerases cannot read and duplicate this information. Thus, the genetic information stored in XNA is "invisible" and therefore unusable for natural organisms based on DNA.
Research history
The structure of DNA was discovered in 1953. XNA's exotic DNA-like structures were first created in the early 2000s. However, research on XNA only gained momentum when it was possible to develop a special polymerase enzyme that can copy XNA from a DNA template and also copy XNA back into the DNA. For example, Pinheiro et al. In 2012 such an XNA-capable polymerase was found and patented, which can work with sequences of approx. 100 bp in length. The study of the production and application of XNA has created the field of xenobiology (as a sub-discipline of synthetic biology). Research is currently ongoing into the development of synthetic polymerases to transform XNA.
More recently, Philipp Holliger and Alexander Taylor (both University of Cambridge) have succeeded in producing so-called XNAzyme . These are XNA equivalents to the natural ribozymes and artificial deoxyribozymes , which act like enzymes as bio- catalysts . This shows that XNAs not only store hereditary information, but can also act catalytically, increasing the possibility that life might once have started with nucleic acids other than RNA or DNA.
construction
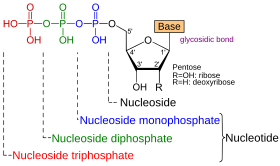
DNA and RNA strands are formed by stringing together so-called nucleotides as building blocks that are too long molecular chains. A nucleotide consists of three chemical components:
- a phosphate
- a sugar group with five carbon atoms ( pentose ): ribose in R NA or its derivative deoxyribose in D NA
- and one of five standard bases : adenine (A) , guanine (G) , cytosine (C) , and uracil (U) for RNA and thymine (T) for DNA
The molecules that make up the six xeno-nucleic acids from Pinheiro et al. (December 2011) are almost identical to those of DNA and RNA, with one exception: In XNA nucleotides, the deoxyribose and ribose sugar groups of DNA and RNA have been replaced by other chemical structures. The phosphate backbone and usually the bases are the same. These substitutions make XNAs functionally and structurally analogous to DNA and RNA, although they are unnatural and artificial. However, as with RNA or DNA, the bases can also be modified, for example to pyrimidine .
Types of synthetic XNA created to date include:
- 1,5-anhydrohexitol nucleic acid ( English hexose nucleic acid , HNA)
- Cyclohexene nucleic acid ( English cyclohexenyl nucleic acid , CeNA)
- Threonucleic acid , more precisely: α-l-threose nucleic acid ( English threonucleic acid, threose nucleic acid , TNA)
- Glycolnukleinsäure ( English glycol nucleic acid , GNA) with ethylene glycol
- Peptide nucleic acid ( English peptide nucleic acid , PNA) with one pseudopeptide ', no phosphate
- 2'-Deoxy-2'-fluoro-arabino-nucleic acid ( English 2'-Deoxy-2'-fluoro-arabinonucleic acid , FANA) and 2'-Deoxy-arabino-nucleic acid ( English 2'-Deoxy-arabinonucleic acid , ANA ), see arabinosyl nucleosides .
- L-aTNA, D-aTNA and SNA ( English acyclic L / D-threoninol nucleic acid, serinol nucleic acid ) with the nitrogen-containing alcohols threoninol and serinol, respectively .
A special case with additional bonds on the sugar group are
- bridged nucleic acids ( English locked nucleic acid, inaccessible RNA , LNA), in contrast to nucleic acids unbridged ( English unlocked nucleic acid , UNA)
| Nucleic acid building blocks of xenonucleic acids vs. RNA / DNA | |
|---|---|

|
|
| Basic structure of an artificial XNA nucleotide - here with a hexose as a sugar |
Basic structure of a natural nucleotide - with ribose (RNA) or deoxyribose (DNA) as sugar |

|

|
| The Glycolnukleinsäure (GNA) (left) is an example of a xeno nucleic acid because it another backbone ( English backbone ) as the DNA (right) has | Different sugar substituents used in XNAs compared to conventional, biological DNA and RNA |
|

|
| 2 ', 3'-Ddidesoxyadenosinetriphosphate (ddATP) as an example of a dideoxynucleotide that for DNA sequencing according to Sanger be used | Deoxyadenosine monoarsenate (dAMAs) as an example of a substituted phosphate group (discussed as naturally occurring in GFAJ-1 bacteria from 2010 ) |
Properties and potential uses
XNA can interact with natural nucleic acid, but is much more stable against nucleic acid degradation mechanisms , since there are no natural enzymes that are suitable for the degradation of hexose-based nucleotides. This would allow this form of genetic information carrier to be used to mark viral or bacterial genomes or genome segments.
HNA could potentially find use as a drug because it can recognize and bind to certain sequences. Scientists have been able to isolate HNAs for binding sequences that target HIV.
Cyclohexene nucleic acids (CeNAs) with stereochemistry similar to D-form can form stable duplexes with themselves and with RNA, whereas the duplexes formed by CeNAs with DNA are less stable.
XNA can also be used as a catalyst, similar to how RNA can act as an enzyme (ribozyme). It has been shown that XNA can cleave and ligate DNA, RNA and other XNA sequences, with most of the activities being XNA-catalyzed reactions on XNA molecules themselves. With the help of this research it could be decided whether the roles of DNA and RNA in life arose through natural selection processes or whether it is a more random occurrence.
See also
- Xenobiology
- DNA: non-standard bases
- Dideoxyribonucleoside triphosphates (ddNTPs): Artificial intermediates in DNA sequencing according to Sanger .
- Deoxyadenosine mono-arsenate (dAMAs) see GFAJ-1 §Discussion about the incorporation of arsenic in biomolecules (questionable incorporation in DNA in Halomonas species GFAJ-1)
Web links
- Nina Weber: Researchers create artificial DNA alternatives . Spiegel online, April 19, 2012.
- Synthetic Biology - What is it?
Individual evidence
- ^ Markus Schmidt: Synthetic Biology . John Wiley & Sons, May 9, 2012, ISBN 978-3-527-65926-5 , pp. 151– (accessed March 6, 2019).
- ↑ a b c Vitor B. Pinheiro et al. : Synthetic Genetic Polymers Capable of Heredity and Evolution . In: Science . 336, No. 6079, 2012, pp. 341-344. bibcode : 2012Sci ... 336..341P . doi : 10.1126 / science.1217622 . PMID 22517858 . PMC 3362463 (free full text).
- ↑ a b Robbie Gonzales: XNA Is Synthetic DNA That's Stronger than the Real Thing . April 19, 2012. Retrieved March 7, 2019.
- ↑ a b Markus Schmidt: Xenobiology: A new form of life as the ultimate biosafety tool . In: BioEssays . 32, No. 4, April 2010, pp. 322-331. doi : 10.1002 / bies.200900147 . PMID 20217844 . PMC 2909387 (free full text).
- ↑ Patent WO2013156786A1 : Polymerase capable of producing non-dna nucleotide polymers. Published on October 24, 2013 , Inventors: Chris Cozens, Philipp Holliger, Vitor Pinheiro.
- ^ World's first artificial enzymes created using synthetic biology . 1st December 2014.
- ↑ Feng Li Sanjay Sarkhel, Christopher J. Wilds et al. : 2′-Fluoroarabino- and Arabinonucleic Acid Show Different Conformations, Resulting in Deviating RNA Affinities and Processing of Their Heteroduplexes with RNA by RNase H , in: Biochemistry, April 4, 2006 Apr, 45 (13), pp. 4141-4152, doi: 10.1021 / bi052322r . PMC 2553321 (free full text)
- ↑ Mike McCrae: Life's First Genes May Have Contained a Nucleic Acid You've Probably Never Heard Of , on: ScienceAlert / Nature of January 20, 2020
- ↑ Keiji Murayama, Hiromu Kashida, Hiroyuki Asanuma: Acyclic L-threoninol nucleic acid (L-aTNA) with suitable structural rigidity cross-pairs with DNA and RNA , in: Chemical Communications Issue 30, 2015, doi: 10.1039 / C4CC09244A
- ^ A b Adele Alagia, Montserrat Terrazas, Ramon Eritja: Modulation of the RNA Interference Activity Using Central Mismatched siRNAs and Acyclic Threoninol Nucleic Acids (aTNA) Units , in: Molecules 2015, 20 (5), pp. 7602-7619 doi: 10.3390 / molecules20057602 , PDF , Fig. 1
- ↑ External identifiers or database links for L-Threoninol : CAS number: 3228-51-1, EC number: 803-664-7, ECHA InfoCard: 100.230.446 , PubChem : 2033049 , ChemSpider : 1534111 , Wikidata : Q27273483 .
- ↑ Niels Langkjær, Anna Pasternak, Jesper Wengel: UNA (unlocked nucleic acid): A flexible RNA mimic that allows engineering of nucleic acid duplex stability. In: Bioorganic & Medicinal Chemistry . Volume 17, No. 15, 2009, pp. 5420-5425, doi : 10.1016 / j.bmc.2009.06.045 .
- ↑ M. A. Campbell, J. Wengel: Locked vs. unlocked nucleic acids (LNA vs. UNA): contrasting structures work towards common therapeutic goals. In: Chemical Society reviews. Volume 40, No. 12, December 2011, pp. 5680-5689, doi : 10.1039 / c1cs15048k , PMID 21556437 (review).
- ↑ Andy Extance: polymer evolution perform non-DNA . April 19, 2012. Retrieved March 6, 2019.
- ↑ Ping Gu, Guy Schepers, Jef Rozenski, Arthur Van Aerschot, Piet Herdewijn: Base Pairing Properties of D- and L-Cyclohexene Nucleic Acids (CeNA) . In: Oligonucleotides . 13, No. 6, 2003, pp. 479-489. doi : 10.1089 / 154545703322860799 . PMID 15025914 .
- ↑ Alexander I. Taylor, Vitor B. Pinheiro, Matthew J. Smola, Alexey S. Morgunov, Sew Peak-Chew, Christopher Cozens, Kevin M. Weeks, Piet Herdewijn, Philipp Holliger: Catalysts from synthetic genetic polymers . In: Nature . 518, No. 7539, 2015, pp. 427-430. bibcode : 2015Natur.518..427T . doi : 10.1038 / nature13982 . PMID 25470036 . PMC 4336857 (free full text).