Meisenheimer rearrangement
The Meisenheimer rearrangement is a rearrangement and name reaction and named after its discoverer, the German chemist Jakob Meisenheimer (1876–1934). In the reaction, an amine oxide is converted into the corresponding alkoxyamine . The reaction can proceed via a [1,2] or a [2,3] rearrangement.
[1,2] rearrangement:
[2,3] rearrangement:
mechanism
[1,2] rearrangement
The Meisenheimer rearrangement takes place via a [1,2] rearrangement when one of the substituents of the tertiary amine oxide is able to stabilize the radical that is formed. This rearrangement takes place via a homolytic mechanism consisting of dissociation and subsequent recombination .
Most of the radicals R 1 and R 2 are alkyl or aryl groups and R 3 are benzyl or diphenylmethyl groups (radical stabilizing). The (C) RN bond is cleaved homonymically, so that two radicals are present that can be detected by ESR or CIDNP .
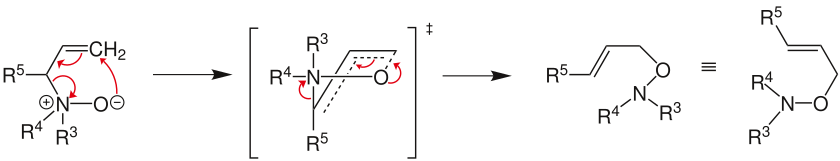
[2,3] rearrangement
The Meisenheimer rearrangement takes place via a [2,3] rearrangement if one of the substituents on the tertiary amine oxide is an allyl group . This rearrangement takes place via a five-membered transition state.
Frequent radicals are: R 5 = H, alkyl or aryl groups and R 3 and R 4 = alkyl or aryl groups.
Individual evidence
- ↑ J. Meisenheimer: About a strange rearrangement of the methyl-allyl-aniline-N-oxide , Chem. Ber. 52, 1667 (1919) doi : 10.1002 / cber.19190520830 .
- ^ March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure Michael B. Smith, Jerry March Wiley-Interscience, 5th edition, 2001, ISBN 0-471-58589-0 .
- ^ László Kürti and Barbara Czakó .: Strategic Applications of Named Reactions in Organic Synthesis: Background and Detailed Mechanisms , Elsevier Academic Press, 2005, pp. 282–283, ISBN 978-0-12-429785-2 .
literature
- Michael, B Smith, Jerry March: Advanced Organic Chemistry. 4th edition, John Willey & Sons, p. 1102
- Jie Jack: Name Reactions. 1st edition, Springer-Verlag, p. 230