Photosynthetic reaction center
The photosynthetic reaction center is the central component of all organisms that carry out photosynthesis . In each case it is a protein complex made up of several subunits. The absorbed light energy leads to a charge separation. Electrons created in this way, which have a much higher redox potential , are then diverted from the reaction center and, after many intermediate steps, used to produce hydrocarbon compounds. The reaction center has hardly changed in the course of evolution, whereas the light-harvesting complexes that surround it have taken on various forms. In more modern organisms, i.e. plants, algae and cyanobacteria , the reaction center is included in a larger complex, the photosystems . The reaction center can be isolated in bacteria. In the following section, the function of the reaction center is explained using the reaction center of the purple bacterium Rhodopseudomonas viridis as an example .
functionality
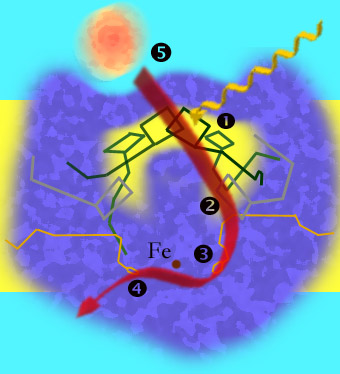
1 : excitation of electrons; 2 : transfer of electrons to a phaeophytin molecule; 3 : The electrons are taken over by an ubiquinone molecule; 4 : Transfer of electrons to the second ubiquinone molecule; 5 : Cytochromes (orange) fill the electron gap in the positively charged chlorophyll of the special pair .
The reaction center of purple bacteria is a complex of three protein chains and various pigments anchored in the plasma membrane and is surrounded by a ring-shaped light- harvesting complex (LH1). The three subunits are identified by the letters L, M and H, with the latter protruding from the membrane. The approximately symmetrical L and M units are located in the membrane.
Both the L and M units each contain two bacteriochlorophylls , as well as a phaeophytin and an ubiquinone . The ubiquinone molecule of the M unit can detach from the complex. Depending on a bacteriochlorophyll molecule of the L and the M-Unit are close together and form a so-called "special pair" ( special pair ), at which the charge separation occurs. The bacteriochlorophyll is used to expand the absorption spectrum, the maximum is shifted depending on the type in areas of longer-wave light, e.g. B. in the P870 of Rhodobacter sphaeroides to 870 nm and in the P960 of Rhodopseudomonas viridis to 960 nm.
One of the two bacteriochlorophyll molecules of the special pair is usually excited indirectly by absorbing the energy from the antenna pigments, which results in charge separation. The phaeophytin molecule of the L unit accepts an electron and is thereby reduced to BPh - . After 200 · 10 −12 seconds the firmly bound ubiquinone molecule takes over the electron. After 200 × 10 -6 seconds, the electron goes to the ubiquinone molecule of the M-unit, which, after it has obtained in the same manner, a second electron leaves the reaction center and the electrons to the cytochrome bc 1 complex transported .
The missing electron of the special pair is replenished by a cytochrome c , which docks to the reaction center.
The task of the H unit, which has no pigments, is still unclear today (2006).
Hartmut Michel , Johann Deisenhofer and Robert Huber received the Nobel Prize for Chemistry for their research into the reaction center of the purple bacterium Rhodopseudomonas viridis , which carries out anoxygenic photosynthesis .
See also
Individual evidence
- ↑ Couglas C. Youvan and Barry L. Marrs: Molecular Mechanisms of Bacterial Photosynthesis . In: Spectrum of Science. Born in 1987, No. 8, p. 62 ff.
- ↑ Information from the Nobel Foundation on the award ceremony in 1988 to Hartmut Michel, Johann Deisenhofer and Robert Huber (English)