photosynthesis

The photosynthesis ( ancient Greek φῶς Phos , German 'light' and σύνθεσις Synthesis , German , composition ' also photosynthesis written) is a physiological process for the production of energy-rich biomolecules from lower-energy substances using light energy. It is powered by plants , algae and some bacteria . In this biochemical process using is of light-absorbing dyes such as chlorophyll light energy into chemical energy converted. This is then used to build energy-rich organic compounds (primarily carbohydrates ) from low-energy inorganic substances ( carbon dioxide (CO 2 ) and water (H 2 O)). Since the energy-rich organic substances become components of the living being, their synthesis is called assimilation .
A distinction is made between oxygenic and anoxygenic photosynthesis. In the case of oxygen, molecular oxygen (O 2 ) is generated. Anoxygenic, which is only used by some bacteria, produces other inorganic substances instead of oxygen, such as elemental sulfur ( S ).
Photosynthesis is the only biochemical process in which light energy, mostly solar energy , is converted into chemically bound energy. Almost all heterotrophic living beings (not capable of photosynthesis) depend indirectly on it, as they ultimately owe their nourishment to it and also the oxygen necessary for energy production through aerobic respiration . The protective ozone layer is also created from the oxygen .
The UVB- dependent formation of cholecalciferol (vitamin D) is also known as photosynthesis.
overview
Photosynthesis can be divided into three steps:
- First the electromagnetic energy is absorbed in the form of light of a suitable wavelength using dyes (chlorophylls, phycobilins , carotenoids ).
- Directly after this, in the second step, the electromagnetic energy is converted into chemical energy by transferring electrons , which have been brought into an energy-rich state by the light energy ( redox reaction ) (see phototrophy ).
- In the final step this chemical energy is used for the synthesis of energetic organic compounds in living beings both constructive metabolism for the growth as in energy metabolism are used for the recovery of energy.
The first two steps are called the light reaction and occur in plants in photosystem I and photosystem II . The last step is a largely light-independent reaction.
The synthesis of energy-rich organic substances is mainly based on the carbon compound carbon dioxide (CO 2 ). This has to be reduced for the recovery of CO 2 . The electrons of oxidizable substances serve as reducing agents (reductants, electron donors): water (H 2 O), elementary, molecular hydrogen (H 2 ), hydrogen sulfide (H 2 S), divalent iron ions (Fe 2+ ) or simple organic ones Substances (such as acids and alcohols , e.g. acetate or ethanol ). In addition, the electrons can also be obtained from the oxidation of simple carbohydrates. Which reductant is used depends on the organism, on its enzymes that are available to it to use the reductants.
| Electron donation (at) or | Photosynthesis form | Occurrence |
|---|---|---|
| Iron-II-ions (Fe 2+ ) | anoxygenic photosynthesis | Purple bacteria |
| Nitrite (NO 2 - ) | anoxygenic photosynthesis | Purple bacteria |
| elemental sulfur (S 0 ) | anoxygenic photosynthesis | Purple bacteria |
| Hydrogen sulfide (H 2 S) | anoxygenic photosynthesis | green non-sulfur bacteria , green sulfur bacteria , purple bacteria |
| Thiosulfate (S 2 O 3 2− ) | anoxygenic photosynthesis | Purple bacteria |
| Water (H 2 O) | oxygenic photosynthesis | Cyanobacteria , plastids of the phototrophic eukaryotes |
| Hydrogen (H 2 ) | anoxygenic photosynthesis | green non-sulfur bacteria |
Photosynthetic balance
In the case of CO 2 as the starting material, the overall reaction scheme of photosynthesis can be formulated in a general and simplified manner using the following sum equations, in which <CH 2 O> stands for the energy-rich organic substances formed.
With a reductant that reduces by releasing hydrogen (H), such as water (H 2 O), hydrogen sulfide (H 2 S) and elementary, molecular hydrogen (H 2 ), (all symbolized here with the general expression <H>) :
With a reductant that reduces (e - ) by releasing electrons , such as divalent iron ions (Fe 2+ ) and nitrite (NO 2 - ):
Some bacteria use organic compounds as reductants, such as lactate, the anion of lactic acid :
The overall reaction of photosynthesis with water or hydrogen sulfide as the reductant can also be formulated by the following general, simplified sum equation:
As a general formulation, H 2 A stands for the reductant H 2 O or H 2 S.
All algae and green land plants only use water (H 2 O) as the reductant H 2 A. Cyanobacteria also mainly use water as a reductant. In this case, the letter A stands for the oxygen (O) bound in the water. It is released as an oxidation product of the water during the so-called oxygenic photosynthesis as elementary, molecular oxygen (O 2 ). All of the oxygen in the earth's atmosphere and hydrosphere is formed through oxygenic photosynthesis .
The photosynthetic bacteria ( Chloroflexaceae , Chlorobiaceae , Chromatiaceae , Heliobacteria , Chloracidobacterium ) can use a much larger spectrum of reductants, but they mainly use hydrogen sulfide (H 2 S). Many cyanobacteria can also use hydrogen sulfide as a reductant. Since in this case A stands for the sulfur bound in the hydrogen sulphide, elemental sulfur (S) and no oxygen is released in this type of bacterial photosynthesis. This form of photosynthesis is therefore called anoxygenic photosynthesis .
Some cyanobacteria can also use divalent iron ions as a reductant.
Even if different reductants are used in oxygenic and anoxygenic photosynthesis, both processes have in common that electrons are obtained through their oxidation . By using these electrons brought to a high energy level (low redox potential ) with light energy, the high-energy compounds ATP and NADPH are formed, by means of which high-energy organic substances can be synthesized from CO 2 .
The carbon required for the synthesis of the energy-rich organic compounds can be obtained from carbon dioxide (CO 2 ) or from simple organic compounds (e.g. acetate). In the first case, one speaks of photoautotrophy . The vast majority of phototrophic organisms are photoautotrophic. The photoautotrophic organisms include z. B. all green land plants and algae. With them, a phosphorylated triose is the primary synthesis product and serves as the starting material for the subsequent construction of building materials and reserve materials (i.e. various carbohydrates). Photoautotrophs drive almost all existing ecosystems (directly and indirectly) with their photosynthesis metabolism , as they provide other living beings with energy-rich building materials and energy sources by building organic compounds from inorganic CO 2 . If simple, organic compounds are used as starting materials, this process, which only occurs in bacteria, is called photoheterotrophy .
Research history
Since ancient times ( Aristotle ) the idea prevailed that the plant takes its food from the earth. It was not until 1671 that Marcello Malpighi subjected this view to an experimental examination, where he came to the conclusion that the nutrient juice in the leaves is processed ("boiled out") by the power of sunlight and only then can it cause growth. After the discovery of oxygen in the 1770s, Jan Ingenhousz showed in 1779 that it is formed in green leaves when they are exposed to light. In another publication in 1796, he stated that the plant takes the carbonic acid (carbon dioxide) it has absorbed as food and "exhales" the oxygen.
Despite these findings, the humus theory was able to hold up into the middle of the 19th century because most researchers were convinced that living things can only emerge from living things. It was only through Justus von Liebig's (1840) successes with mineral fertilizers that it became indisputable that plants can assimilate inorganic substances. In the 1860s, Julius von Sachs described that chloroplasts accumulate starch in light , which is believed to be formed from sugar as the primary product of photosynthesis.
How the assimilation of carbon dioxide takes place and how this process is related to the action of light remained unclear for a long time. In addition to the assumption that the carbon dioxide is photolytically split directly by the chlorophyll , Frederick Blackman and Gabrielle Matthaei postulated in 1905 that a distinction had to be made between a photochemical light reaction and an enzymatic dark reaction. In 1930, Cornelis Bernardus van Niel suggested, in analogy to his results with sulfur bacteria , that photosynthesis was an exchange of hydrogen between a donor and carbon dioxide as an acceptor, the donor being water (analogous to H 2 S for sulfur bacteria ). Robert Hill provided impressive evidence for these theses in 1937 when he reported that isolated chloroplasts form oxygen even in the absence of carbon dioxide when iron salts are present as artificial electron acceptors ( Hill reaction ). In the course of the 1950s, numerous researchers elucidated the details of the light and dark reactions.
Absorption of light energy
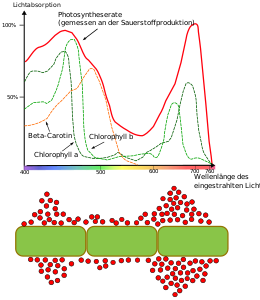
below : Engelmann bacteria experiment (graphic not to scale). The light spectrum of a prism was projected onto a thread thallus of a green alga ( Oedogonium ). A particularly large number of aerophilic, actively swimming bacteria (red spheres) accumulate in the red and blue areas. Due to the shielding effect of the carotenoids in the blue area, the photosynthetic spectrum of activity is smaller there than in the red area.
In phototrophic organisms, the energy of light is captured by dyes. In green plants and cyanobacteria it is chlorophylls , in other bacteria it is bacteriochlorophylls. Light from different wavelength ranges is absorbed by these dyes. The so-called green gap leads to the characteristic green color. The optimal spectral range for photosynthesis was determined experimentally for the first time by Engelmann's bacterial experiment. The light-absorbing dyes are also called chromophores . If these form complexes with surrounding proteins, these are also known as pigments .
When light hits a pigment, the chromophore changes to an excited state. Depending on how the conjugated double bonds of the chromophore are built, the energy for such an excitation and thus the absorption spectrum differ. In the case of chlorophylls a and b occurring in plants , mainly blue and red light are absorbed, while green light is not. The chlorophyll excited by light can now transfer its excited electron to another substance, an electron acceptor; a positively charged chlorophyll radical (Chl • + ) remains. The transferred electron can finally return to the chlorophyll radical via an electron transport chain via further electron carriers. In this way the electron translocates protons through the membrane ( proton pump ), so the light energy is converted into an electrical and osmotic potential ( chemiosmotic coupling ).
Light harvesting complexes

Photosynthesis using simple pigments would be relatively inefficient, as these would only oppose the light over a small area and would also only absorb in a narrow range of wavelengths. By arranging light-collecting complexes containing chlorophyll to form antennas around a common reaction center, both the cross-section is enlarged and the absorption spectrum is broadened. The closely spaced chromophores in the antennae transmit the light energy from one pigment to another. This defined amount ( quantum ) of excitation energy is also called exciton . The excitons finally reach the reaction center in a few picoseconds. The exciton transfer presumably takes place within a light-harvesting complex by delocalized electrons and between individual light-harvesting complexes without radiation by the Förster mechanism .
In plants, the light-harvesting complexes form a central antenna ( core ) and an outer antenna and are embedded in the thylakoid membrane together with the reaction center . Not only chlorophyll a and b serve as chromophores , but also carotenes and xanthophylls . On the one hand, these protect the antenna if a chlorophyll molecule develops a harmful triplet state . On the other hand, these chromophores broaden the range of wavelengths for capturing light.
In cyanobacteria, the antennae are superposed on the thylakoid membrane from outside. The antenna complexes are called phycobilisomes , the phycobilin proteins of which absorb green light in particular.
Green sulfur bacteria and green non-sulfur bacteria use so-called chlorosomes for their anoxygenic photosynthesis . These are elongated, lipid-like particles that lie on the cytoplasmic side of the membrane and are connected to the photosynthetic reaction center. They are particularly effective light collectors.
Oxygen photosynthesis

The green plants, algae and cyanobacteria use the energy of light to store energy in the form of adenosine triphosphate (ATP) and to gain electrons from water as a reducing agent. Water (H 2 O) is oxidized by withdrawing electrons from it, and in the process molecular oxygen (O 2 ) and protons (H + ) are released ( photolysis of water). This form of photosynthesis is called oxygenic photosynthesis because of the release of oxygen ( oxygenium ) . The electrons obtained are transferred to the final acceptor NADP + via a series of electron carriers in the thylakoid membrane , which are necessary in the organism's metabolism, especially for the build-up of carbohydrates (“dark reaction”).
- Partial equation splitting of water by light
During this process, protons are simultaneously transported into the lumen of the thylakoids. The resulting proton gradient ΔP (electrochemical gradient) drives the enzyme ATP synthase via chemiosmotic coupling , which forms and regenerates ATP from ADP and phosphate (photophosphorylation):
- Partial equation of ATP synthesis, with water being released
The number of ATP molecules of 3 ATP per 2 H 2 O given above results indirectly from the energy requirement of the Calvin cycle (“dark reaction”). It is not known whether exactly three molecules of ATP are actually formed from the splitting of two molecules of water. In order to be able to oxidize water on the one hand and to be able to reduce NADP + on the other hand , two different photo systems are connected in series, the redox potentials of which are changed by the absorption of light. Photosystem II provides a strong oxidizing agent to oxidize water, while photosystem I generates a strong reducing agent to reduce NADP + . This reaction is commonly referred to as the "light reaction" as this part of photosynthesis is directly dependent on light.
In the overall balance, one molecule of oxygen is formed in the light reaction, two molecules of NADP are reduced to NADPH and about three molecules of ADP are phosphorylated to ATP:
- Partial equation "light reaction"
Or more generally for the splitting of 12 water molecules:
- Simplified partial equation "light reaction", [H] represents a reduction equivalent
The reducing agent (NADPH) obtained in the light reaction and the energy source (ATP) obtained in the process are then used in the so-called Calvin cycle ("dark reaction"), in which carbohydrates are built up from carbon dioxide. The NADPH is used for the reduction of 1,3-bisphosphoglycerate to glyceraldehyde-3-phosphate .
- simplified partial equation "dark reaction"
The released oxygen does not come from the fixed CO 2 , but from water. Therefore, in the first sum equation below, there are 12 water molecules on the left and 6 O 2 molecules on the right . In the overall equation of oxygenic photosynthesis, glucose (C 6 H 12 O 6 ) serves as an example, it represents the primary product:
- simplified gross reaction equation for oxygenic photosynthesis
- simplified net reaction equation for oxygenic photosynthesis
- Dextrose ( glucose ) and oxygen are produced from carbon dioxide and water through the addition of energy (light) .
- Word equation for oxygenic photosynthesis
Oxygen photosynthesis is carried out by cyanobacteria and all eukaryotic phototrophic organisms. In addition to all green plants, examples of this are also numerous single-celled algae ( protists ). The importance of this process lies in the primary production of organic substances, which are used by chemoheterotrophic organisms as a source of energy and building materials, and in the formation of oxygen, which is essential for all aerobic organisms and which is formed on earth almost exclusively through oxygenic photosynthesis.
Linear (non-cyclic) photophosphorylation
In organisms with oxygenic photosynthesis, the two membrane-integrated photosystems II and I are connected in series. Similar to the respiratory chain, the two photosystems are linked by an electron transport chain which, in addition to the small molecule plastoquinone, also includes a further membrane-integrated protein complex ( cytochrome b 6 f complex ) and the small protein plastocyanin . If the redox potentials of all the redox partners involved in the reaction are plotted, a kind of zigzag curve is produced, which is reminiscent of a rotated “Z” (Z diagram, see figure).
The excitation energy required for electron transfer in the reaction centers of the photosystems is absorbed in the form of radiation primarily by the light-collecting complexes , which contain chlorophyll- a , chlorophyll- b and carotenoids , which absorb in a wide range of the visible wavelength spectrum (see above spectrum ). When a red light quantum is absorbed, the molecule changes to the excited energy state S 1 , when a blue light quantum is absorbed into the state S 2 with higher energy. Only the S 1 state can be used for photochemistry. However, by releasing the excess energy as heat (internal conversion) , it is possible to switch from S 2 to S 1 level, which also makes higher-energy light quanta usable.
Through radiationless energy transfer , the energy of the excited states can be transferred between closely neighboring chlorophyll molecules to the reaction centers of the photosystems. The fallback to the initial level S 0 takes place with the release of energy, the type of release being dependent on the efficiency of the respective process.
With a quantum yield of about 85% of the excitation energy, a charge separation is carried out in the open reaction centers of photosystem II, in which an electron is transferred from the paired chlorophyll a molecules ( special pair , P680 ) to a primary acceptor, a pheophytin (Phe) D1 subunit, is transferred. From there it passes through the firmly bound plastoquinone Q A (D2 subunit) to a loosely bound plastoquinone (Q B ) (D1 subunit). After the absorption of two electrons and protonation by H + from the stroma , Q B is released as plastoquinol ( also plastohydroquinol, PQH 2 ) into the membrane matrix, in which it can freely diffuse. A recently discovered third plastoquinone (Q C ) mediates the exchange of Q B with the quinone pool of the membrane.
The remaining oxidized P680 • + radical, which is a very strong oxidizing agent with a redox potential of more than +1 V, is reduced by a tyrosine residue (Tyr z ). This in turn is regenerated by the manganese cluster of the water-splitting complex .
PQH 2 diffuses in the thylakoid membrane to the cytochrome b 6 f complex. The complex plays a central role in the electron transport chain and mediates two successive electron transitions. The first electron is withdrawn from PQH 2 by the Rieske protein , a 2-iron-2-sulfur protein . This protein owes its name to the discoverer John S. Rieske , who isolated the protein with colleagues in 1964. The Rieske protein passes the electron on to the cytochrome f of the cytochrome f subunit. The cytochrome f in turn donates an electron to a plastocyanin . When the electron is picked up, the plastocyanin is located on the side of the cytochrome b6f complex facing the lumen.
The second electron transfer takes place via the membrane-integral b subunit of the cytochrome b 6 complex which contains two cytochromes of the b type. These transfer the second electron from the semiquinone radical PQH • - to a plastoquinone, which is protonated by H + from the stroma (Q cycle). The PQH 2 reoxidation on the cytochrome b 6 f complex is the slowest and thus rate-limiting step in the electron transport chain with a duration of about 5 ms. This is probably due to the necessary change in conformation of the Rieske protein and the restricted diffusion of PQH 2 to the active center of the complex, which is located in a deeply sunk pocket.
In sum, PQH 2 is reoxidized to PQ, an electron is recycled in the Q cycle and an electron is finally transferred to the protein plastocyanin (PC), which can accept an electron. During this transfer, one proton per electron is translocated from the stroma of the chloroplasts into the thylakoid lumen.
Plastocyanin is a water - soluble, copper - containing protein, the copper atom of which changes between the oxidation states Cu I and Cu II and can thus accept and release an electron. It diffuses in the lumen of the thylakoid. In terms of its function, it is similar to cytochrome c in the respiratory chain. In some cyanobacteria and algae, plastocyanin is replaced by the variant cytochrome c 6 .
The reduced plastocyanin released by the Cyt- b 6 f complex finally reaches the photosystem complex I (PS I). PS I also contains a pair of chlorophyll molecules and has an absorption maximum at around 700 nm and a redox potential E ' 0 = + 0.45 V. Like P680 in PS II, the chlorophyll a pair in the reaction center is put into an energetically higher state ( E ' 0 = −1.3 V) and releases an electron. This creates a positive chlorophyll radical (Chl-a • + ), which accepts an electron from the docked plastocyanin and is thereby reduced to Chl- a . After releasing the electron, PC can be reduced again by the Cyt- b 6 f complex.
The electron that was given up by the chlorophyll- a molecule first hits a first acceptor, A 0 . It is assumed that this is a special chlorophyll. This is an unusually strong reducing agent and reduces a tightly bound phylloquinone (Q, also A 1 ). From there the electron is transferred to an iron-sulfur center (F x ) and finally arrives at ferredoxin (Fd) via further iron-sulfur centers (F A , F B ). This is located on the stromal side of the thylakoid membrane. The reduced Fd binds to a ferredoxin-NADP reductase and reduces NADP + to NADPH.
The electron transport is coupled to a proton translocation from the stroma into the lumen. For every electron transferred completely from water to NADPH, three protons are translocated into the lumen. This creates a proton concentration difference (ΔpH) as well as an electric field across the thylakoid membrane, which in total is called the proton motor force Δμ H + ( proton motive force ). According to Peter Mitchell's chemiosmotic theory, the proton motor force is used by the ATP synthase to generate three ATP from ADP and inorganic phosphate with the help of 14 protons . This process is also called photophosphorylation . In the balance, three protons are transported per electron due to the linear electron transport, taking into account the Q cycle. Since 14 protons are not needed to generate three ATP, ATP and NADPH are not generated in a ratio of 3: 2 = 1.5, but in a fixed ratio of 9: 7 = 1.3.
Oxygen producing complex

The electron gap of the chlorophyll radical in the reaction center of PS II has yet to be closed. The electrons are obtained from water (E ' 0 = + 0.82 V). A tyrosine residue of the D1 subunit (Tyr161 = Tyr Z ) and a manganese cluster are involved in this “water splitting ”. The Chl a radical withdraws an electron from the reactive tyrosine residue, whereby it is itself oxidized to a tyrosine radical. In order for the tyrosine radical to be reduced again, it needs an electron from a special metal complex , the manganese-calcium cluster. The manganese-calcium cluster (Mn 4 CaO 5 ) is the most important component of the oxygen-producing complex (“ oxygen-evolving complex ”, OEC). The cluster is essentially made up of four manganese atoms, one calcium atom and one peripheral chlorine atom . This unusual composition of five metal atoms is extremely rare. Only one example of a CO monohydrogenase is known in which other metal atoms ( Fe , Ni ) have a similar composition via sulfur bridges. Functionally, the calcium atom can be replaced by a strontium atom and the essential chlorine atom by a bromine atom .
The exact geometry of the metal atoms has not yet been fully clarified despite intensive studies. The metal atoms with oxygen atoms may be present as shown in the figure on the right and are complexed by various amino acids of the D1 or CP43 subunit . All manganese atoms are coordinated by six molecules and two molecules of water bind to manganese atom number 4 (Mn4).
The Mn 4 CaO 5 cluster works like a kind of battery . Gradually, three manganese ions give off one electron each and switch between the Mn III and Mn IV oxidation states (see figure). This allows different oxidation states of the cluster (S 0 to S 4 ) to be achieved. However, since the electrons in the cluster are strongly delocalized , it is difficult to state the exact oxidation state of the manganese atoms. It has been discussed that the fourth manganese atom in the S 4 state reaches a formal oxidation state of V. It is more likely, however, that an oxo radical is formed (see figure). In 2014, with the help of a special application of X-rays - free electron X-ray lasers (serial femtosecond X-ray structure analysis), scientists were able to record images of the different excited states of photosystem II. The exact oxidation state has not yet been clearly clarified (as of 2014). It is postulated that the oxygen atom no. 5 (O5) is not present as an oxo ligand (O 2 - ), but as a water molecule during the S 0 state and as a hydroxide ion (OH - ) during the S 1 state. It should also be one of the two water molecules that are used to form oxygen in the course of water splitting.
Only when four electrons have been transferred to the Tyr Z (S 4 ), oxygen is formed and released and the reduced state (S 0 ) is reached again. This cycle is also known as the “Kok cycle”. A total of four excitons are required to release one molecule of oxygen, as the studies by Pierre Joliot and Bessel Kok have shown.
It is believed that a gradual oxidation of the water would liberate many reactive oxygen species (ROS). With the mechanism outlined above, this risk is minimized. The special thing about this enzyme, however, is not the fact that it releases oxygen ( catalases could also do this), but that it forms an O – O bond between two water molecules.
Cyclic photophosphorylation
The cyclic electron transport in the light reaction takes place only at photosystem I . The electrons from ferredoxin are not passed on to the NADP + reductase as in the usual non-cyclic electron transport (see above), but are returned to the cytochrome b 6 f complex. From there the electrons get back to photosystem I and finally fill the electron gap in the reaction center. Although no NADPH is formed in the process, a proton motor force is built up, which is used to produce ATP. Since this is a cycle, this process is also called cyclic photophosphorylation .
C 4 plants have an increased need for ATP and could therefore use cyclic photophosphorylation more intensively than C 3 plants . But the latter are also dependent on cyclic photophosphorylation in order to stoichiometrically cover the NADPH and ATP requirements for the Calvin cycle (including photorespiration).
Pseudocyclic electron transport
In non-cyclic electron transport, ferredoxin (Fd) gives up its electron on NADP + , so that NADPH is formed. Due to the high degree of reduction, there is also the possibility that the electron will be transferred to oxygen (O 2 ). The superoxide radical is created in the so-called Mehler reaction . It was named after the work of Alan H. Mehler:
This radical belongs to the highly reactive oxygen species (ROS) and can damage proteins, membranes and DNA. Therefore, this is first reacted with a superoxide dismutase ( EC 1.15.1.1 ) to oxygen and hydrogen peroxide (H 2 O 2 ) disproportionated . The reaction can also take place spontaneously:
Hydrogen peroxide is also an ROS and is rendered harmless by an ascorbate peroxidase ( EC 1.11.1.11 ) present in the thylakoid membranes . During this detoxification process, ascorbate is oxidized to monodehydroascorbate, while H 2 O 2 is reduced to water. The presence of ferredoxin regenerates monodehydroascorbate back to ascorbate. The balance thus results:
and thus overall:
Normally, four electrons are released from the water splitting at PS II and transferred to ferredoxin. In the course of the Mehler reaction, these are in turn used to form water, so that one speaks of a pseudocyclic electron transport . As in cyclic electron transport, the electrons are not transferred to NADP + . This cycle was in the literature as water-water cycle ( water-water cycle hereinafter): A molecule of water is split and subsequently generates another again. In the overall balance, the water splitting of PS II is formally reversed and no reduction equivalents are built up.
The Mehler reaction occurs v. a. then when there is a lot of reduced NADPH and thus also a lot of reduced ferredoxin. In the case of pseudocyclic electron transport, as in cyclic transport, only one proton gradient is generated in the Q cycle, so that ATP is generated. Under these conditions, however, there is normally no ADP to build up ATP, so that only a high proton gradient occurs. Excess excitation energy can easily be converted into heat. Possibly this serves as an "overflow valve" under high light intensities and protects the photosystem II not only in plants, but also in all photosynthetically active algae and cyanobacteria. However, this assumes that sufficient ascorbate is present.
Two molecules of monodehydroascorbate can, however, also disproportionate to ascorbate and dehydroascorbate . To regenerate dehydroascorbate, glutathione is oxidized, which in turn is reduced by a glutathione reductase ( EC 1.8.1.7 ). NADPH is consumed in the process. Formally, this does not change anything in the overall balance described above.
Regulation of electron transport in oxygenic photosynthesis
If electrons or states of excitation are transferred to oxygen in an uncontrolled manner, damage to the photosynthetic apparatus, membrane systems and proteins can occur. Regulation of electron transport is therefore extremely important. Last but not least, it must also be adapted to the plant's need for NADPH and ATP. Long-term regulatory mechanisms that take place at the transcriptional level are not discussed in this section, but they definitely take place.
Lateral uneven distribution of the photosynthetic complexes in the thylakoid membrane

1: outer envelope
2: intermembrane space
3: inner envelope membrane (1 + 2 + 3: sheath)
4: stroma (matrix)
5: thylakoid lumen (inside the thylakoid)
6: thylakoid membrane
7: Granum (Grana blade)
8: thylakoid (Stromalamelle)
9: Starch body
10: plastid ribosome ( plastoribosome )
11: plastid DNA ( cpDNA )
12: plastoglobulus (spherical structure made of lipids; pl .: plastoglobuli)
The photosynthesis complexes Photosystem II PS II, Cytochrome b 6 f and Photosystem I PS I are embedded in the thylakoid membrane. The photosystems are not evenly distributed laterally. Because of its interaction with the light-harvesting complexes, PS II is located in the stacked areas of the thylakoid ( granal lamellae ), the stromal side of PS I must be freely accessible for NADP + reduction and is therefore located in areas that are exposed to the stroma. These include unstacked areas (stromal lamellae, no. 8 in the right figure), as well as the edge areas of the grana stack ( margins and end membranes ). The ATP synthase also needs space on the stroma side of the membrane and can therefore only be found in these areas.
The spatial separation of the two photosystems should also prevent uncontrolled overflow ( spillover ) of the excitons from the PS-II to the PS-I complex. While excitons flow away from the antennas to the PS I very efficiently ( funnel trap ), the excitation energy can even jump out of the PS II again ( shallow trap ). Therefore, if the two photosynthesis complexes were in direct proximity, the excitons would more frequently be added to PS I at the expense of PS II.
Redistribution of the light collecting complexes LHC-II ( state transitions )
Under certain circumstances, the photosystems can be excited to different degrees because they have different absorption spectra. This state is perceived by the plant via the redox state of the plastoquinone pool.
- In weak light, when the two photosystems are not yet working at their capacity limit, photosystem I PS I is more strongly stimulated than photosystem II PS II. Therefore, there is a redistribution of the light-collecting complexes LHC-II from PS I to PS II, around the uneven stimulation to counteract. This stimulates PS II more strongly.
- If, on the other hand, PS II is stimulated more strongly than PS I, reduced plastoquinol accumulates in the thylakoid membrane. A protein kinase is activated by the changed redox state of the plastoquinone pool . As a result, LHC-II complexes are phosphorylated and migrate from PS II to PS I. This means that PS I is preferred for light excitation.
The redistribution of the LHCs is called state transitions : If all LHC-II are associated with PS II, state 1 is present. However, if they are associated with PS I, state 2 is present ( state 2 ).
Thermal dissipation of excess energy
Under certain circumstances, more NADPH and ATP are produced than can be consumed by the dark reaction. This can be the case, for example, with high light intensity, or with high temperatures or drought stress , when the stomata are closed in order to reduce the loss of water. This also reduces the uptake of CO 2 , so that the dark reaction is limited and slowed down by the CO 2 concentration. At low temperatures, the enzymatic activity of the metabolism in particular is slowed down, but the electron transfers in the light reaction are hardly any, so that ATP and NADPH are produced in excess. Since the electron transport chain does not have an acceptor for the electrons available in these cases, the probability of the formation of reactive oxygen species (ROS), which can damage the photosynthetic apparatus and the cell, increases.
To dissipate the excess energy, zeaxanthin comes into action as part of the xanthophyll cycle . Here, zeaxanthin binds to a subunit of the LHC-II complex and can absorb the energy of excited chlorophyll molecules and release it as heat. About 50–70% of all absorbed photons are converted into heat in this way. Diatoxanthin is used in a similar cycle instead of zeaxanthin for diatoms .
In addition to the above-mentioned reactions, especially in the case of drought stress, the reassimilation of the CO 2 released by photorespiration also contributes to the elimination of excess light energy ; the proportion of the individual processes in the consumption of light energy varies, however, depending on the leaf tissue (palisade or sponge parenchyma) examined Plant species and type of metabolism (C 3 or C 4 plant).
Anoxygenic photosynthesis
Many phototrophic bacteria carry out anoxygenic photosynthesis. A single photosystem is involved, either with a reaction center of plant type I (PS I) or type II (PS II). In contrast to cyanobacteria and phototrophic eukaryotes , the reaction centers contain bacteria chlorophylls. As in oxygenic photosynthesis, this chlorophyll pair ( special pair ) is stimulated by light energy, which causes its redox potential to drop sharply. As a result, the excited bacteriochlorophyll pair surrenders its electron to a primary acceptor. Depending on the reaction center, the first stable electron acceptor is either an iron-sulfur protein (PS I) or a quinone (PS II). From there it is finally passed back to the reaction center via a Q cycle (cyclic electron transport). During this process, a difference in proton concentration is built up, which drives an ATPase . Since ATP is built up but no reducing agent , the latter must be formed from external electron donors (inorganic or organic compounds).
In addition to this cyclic electron transport, there is also a non-cyclic one, through which reducing agents are formed directly. In order to fill the resulting electron gap, external electron donors are used, for example H 2 S, divalent iron ions (Fe 2+ ) or nitrite (NO 2 - ). Elemental sulfur (S), trivalent iron ions (Fe 3+ ) or Nitrate (NO 3 - ) as reaction products.
The redox potential of the bacteriochlorophyll pigment is not sufficient to split water. Accordingly, there is no water-splitting complex (see above). In anoxygenic photosynthesis, water cannot be used as a source of electrons and thus no molecular oxygen can be produced.
Type II anoxygenic photosynthesis
Green non-sulfur bacteria (Chloroflexi) and purple bacteria (purple sulfur bacteria and sulfur-free purple bacteria) use a reaction center of type II in anoxygenic photosynthesis. The absorption maximum of bacteriochlorophyll a or b in the center is 870 nm Standard redox potential E ' 0 drops sharply from +0.6 V to −0.8 V. It donates its electron to a bacteriophaeophytin . From there it finally reaches an associated quinone (Q B , E ' 0 = + 0.0 V) via a firmly bound menaquinone (Q A ). If this is reduced to quinol by two electrons, it leaves the PS II complex and diffuses in the membrane to a cytochrome bc 1 complex. During the Q cycle, the electrons are transferred to this complex, a proton gradient (Δμ H + ) is built up. This operates an ATPase, whereby ATP is formed. From there, the electron in the cytochrome bc 1 complex reaches a cytochrome c (cyt c 2 ), which diffuses back to the reaction center in the cytoplasm, associated with the membrane. There the electron gap is closed by oxidation of the Cyt c 2 , a new cyclic electron transport can begin.
In order to form reducing agents, sulfur-free purple bacteria ( Rhodospirillum , Rhodobacter ) and green non-sulfur bacteria (e.g. Chloroflexus ) preferentially oxidize organic compounds; they usually grow with it photoorganothrophic. Most anoxygenic phototrophic bacteria can also fix (CO 2 ) if they use inorganic electron donors such as hydrogen (H 2 ) or (H 2 S), so they are often facultative photolithoautotrophic.
The quinone can also transfer its electrons to NAD (P) + in a non-cyclic electron transport , which catalyzes an NADH quinone oxidoreductase. However, the redox potential of the quinone is too high to reduce NAD (P) + (E ' 0 = −0.32 V) directly. Therefore, energy in the form of the proton motor force Δμ H + is tapped for this reversed (declining) electron transport . Since the electron has been removed from the cycle, the bacteriochlorophyll a initially remains in the reaction center as a positively charged radical. To fill this gap, electrons from external sources are used. Purple sulfur bacteria (e.g. Chromatium , Ectothiorhodospira ) oxidize H 2 S to sulfur, which is deposited intra- or extracellularly.
In Rhodopseudomonas viridis , a purple bacterium, the kinetics of electron transfer are known. After excitation of the pair of bacteriochlorophylls, the electron reaches the bacteriophaeophytin after 3 ps. From there it is transferred to the menaquinone Q A after 200 ps and from there reaches the ubiquinone Q B relatively slowly (6 µs) . The electron gap in the bacteriochlorophyll pair of the reaction center is closed by cytochrome c 2 after 0.27 µs.
Type I anoxygenic photosynthesis
Green sulfur bacteria (eg. B. Chlorobium ) and also heliobacteria ( Heliobacterium ) have a reaction center of the type I. In the former, there is a bacteriochlorophyll a with an absorption maximum of 840 nm, at heliobacteria a bacteriochlorophyll g with 798 nm before. In the cyclic electron transport, the bacteriochlorophyll a (E ' 0 = + 0.3 V) reaches an energetically higher state (E' 0 = −1.2 V) after being excited by light and releases its electron to another bacteriochlorophyll. From there it reaches ferredoxin (Fd) via a firmly bound menaquinone and various iron-sulfur centers (F x , F A / F B , E ' 0 = −0.5 V). Fd releases its electron to menaquinone located in the membrane, which is fed into a Q cycle. A proton concentration difference is built up on the membrane. Finally, a tetracytochrome unit (Cyt c 553 ) is reduced via a Cyt bc 1 complex , which returns to PS I in order to close the electron gap in the reaction center.
Here, too, there is the possibility of a non-cyclic electron transport: When the electrons are transferred from the iron-sulfur center to a ferredoxin , they get from there to NAD (P) + . This catalyzes a ferredoxin-NADP + oxidoreductase. Since the redox potential of Fd is lower than that of NAD (P) + , this reaction can take place without any expenditure of energy. In order to fill the electron gap that has arisen in bacteriochlorophyll in the non-cyclic electron transport, green sulfur bacteria, for example, oxidize H 2 S to sulfur.
In the case of heliobacteria, there is no known way to fix CO 2 . They do not have any RuBisCO or ATP citrate lyase (for the reductive citric acid cycle ), but ferment organic compounds such as pyruvate , lactate , butyrate and acetate . Some strains can also grow with sugars and with ethanol. So they grow heterotrophically .
Anoxygenic photosynthesis in the deep sea
Most photosynthetically active organisms use incident solar radiation as light energy, which is why photosynthesis mainly takes place on the earth's surface. A green sulfur bacterium that carries out anoxygenic photosynthesis with hydrogen sulfide or sulfur as a reductant was discovered on a black smoker about 2,500 meters deep on the East Pacific ridge . No sunlight reaches the bacteria at this depth. Its extremely light-sensitive chlorosomes are able to absorb the weak infrared radiation from the hydrothermal source and make it usable for photosynthesis.
Carbon dioxide assimilation
The reduction equivalents and ATP obtained in the light reaction are used to build up carbohydrates. The processes taking place in the process are therefore also referred to as the secondary reaction of photosynthesis . Since they are not directly dependent on light, they are also known as the dark reaction . But this name is misleading. It corresponds to the fact that the processes take place separately from the "light reaction" of photosynthesis and do not require any light per se . However, since they need ATP and NADPH from the light reaction, the dark reaction does not take place in the dark and is at least indirectly dependent on the light. In addition, some of the enzymes involved are only active in light.
There are several options for carbon dioxide assimilation:
Calvin cycle
All organisms with oxygenic photosynthesis as well as some microorganisms with anoxygenic photosynthesis (purple bacteria) can fix carbon dioxide in the so-called Calvin cycle . As an essential feature, CO 2 condenses on ribulose 1,5-bisphosphate (RubP), which is catalyzed by the enzyme ribulose 1,5-bisphosphate carboxylase / oxygenase ( RuBisCO ). Glyceraldehyde-3-phosphate (G3P) is built up through the following reductions while consuming NADPH and ATP. In plants, the reactions take place in the stroma of the chloroplasts . The enzymes involved in this process are not sensitive to oxygen. However, RuBisCO tends to use oxygen instead of carbon dioxide ( photorespiration ), which reduces the efficiency of carbon dioxide fixation.
There are three different ways of fixing the carbon dioxide. Most of the plants belong to the type of C 3 plants , the first detectable intermediate product (G3P) of which contains three carbon atoms. C 4 plants have anatomically and metabolically adapted to strong solar radiation and are more efficient in this area. They have a spatially separate CO 2 pre-fixation. CAM plants , on the other hand, can temporally separate the primary CO 2 fixation from the actual photosynthesis and, if there is a lack of water, only open their stomata at night and store the absorbed carbon dioxide in the form of malic acid . After sunrise they close the stomata, release the carbon dioxide from the malic acid and feed it into the Calvin cycle.
Reductive citric acid cycle
In the course of anoxygenic photosynthesis in green sulfur bacteria or green non-sulfur bacteria, CO 2 is assimilated by the reductive citric acid cycle or the hydroxypropionate cycle.
Heliobacteria
Heliobacteria are only photoheterotrophic and not photoautotrophic because they do not grow with CO 2 as the sole carbon source, but require organic carbon sources such as fermentation products. But you can still assimilate CO 2 through several anaplerotic reactions of an incomplete reductive citric acid cycle . They obtain the reducing agent and ATP required for this via an anoxygenic type I photosystem.
Energy balance
There are various parameters for quantifying the effect of photosynthesis in relation to the energy used.
The efficiency is the ratio of the increase in the energy content during the synthesis of the photosynthetic product to the light energy used for photosynthesis, so it is a dimensionless quotient (0–1 or 0–100%). Other parameters for the photosynthesis product (e.g. mass or amount of substance) can also be related to the energy expenditure. The quantities obtained are called effectiveness , the unit of measurement of which corresponds to the quantity selected for the photosynthesis product (e.g. grams per joule or moles per joule).
If the primary effect of the photosynthesis of a chloroplast or a microorganism is to be shown, the energy content (unit of measurement joule), the mass (unit of measurement gram) or the amount of substance (unit of measurement mole) of one of the first synthesis products, usually glucose, is used. For considerations of a whole plant, a plant community , a microorganism population or for global considerations, it is advantageous to use the mass of the total dry biomass formed (or the carbon contained in it).
When it comes to the question of what energy content the available light has, there are various starting points. Either the energy of the entire spectrum of radiation from the sun is taken into account. Or only the part of the spectrum that can be used for photosynthesis in principle is used for the calculations. This portion of the radiation represents the photosynthetically usable radiation (English "photosynthetically active radiation", abbreviation "PAR"). Depending on the assumed wavelength range, the energy of the PAR is 40 to 50 percent of that of the total radiation. In the case of plants and algae - depending on the author - the wavelength range from 360 to 720 nm is assumed, partly from 360 to 700 nm and partly from 400 to 700 nm.
The theoretical efficiency of photosynthesis results from the ratio of the chemical energy obtained to the energy absorbed by the electromagnetic radiation :
Energy balance in plants and other organisms with oxygenic photosynthesis
Efficiency
In order to build up 1 mole of carbohydrate D- glucose from 6 moles of CO 2 in the Calvin cycle , 2872 kJ are required under standard conditions:
The energy required is provided by NADPH + H + and ATP, which are created in the primary reaction by light.
By photolysis of water, 2 moles of NADPH + H + are formed per mole of O 2 . The amount of ATP formed per mole of O 2 depends on the Q cycle and is 2 moles without the Q cycle and 3 moles with a full Q cycle. Based on the formation of one molecule of glucose, 12 moles of NADPH and 12 moles or 18 Moles of ATP are available in the secondary reaction.
The standard molar free enthalpy of formation for NADPH + H + is:
The standard molar free enthalpy of formation for ATP from ADP + P i is:
Therefore the yield of the endergonic light reaction per mole of O 2 is between:
Both and are above the standard molar enthalpy of formation for CO 2 fixation of 2872 kJ / mol.
In the endergonic light reaction, 4 excitons must be absorbed as excitation energy in each of the two photosystems, i.e. at least 4 moles of photons with a wavelength of 680 nm on PS II and at least as many with a wavelength of 700 nm on PS I. The energy of the photons amounts to:
However, a requirement of 9 to 10 photons was determined experimentally on the basis of the quantum yield . Since 6 moles of water are split to form 1 mole of glucose, the electromagnetic energy absorbed is between:
This results in values between:
Since not only photons of the red part of the spectrum radiate in a natural environment - i.e. in sunlight - the actual (gross) efficiency is a maximum of 20%.
Net primary production
The effectiveness of photosynthesis in relation to the total amount of sunlight falling on the earth can be stated as follows:
| Total irradiation | 100% |
| Half of it is absorbed, the rest is let through or reflected on the sheet | 50% |
| 3.2% of this in gross primary production, the rest converted into heat | 1.6% |
| Half of this as net primary production, the other half is consumed in respiration | 0.8% |
So only a small part of the incident solar energy is used by the plant to convert carbon dioxide into cell material in the net primary production. Since part of the energy is used to maintain the metabolism, the (gross) efficiency of 20% estimated above is reduced even further. Therefore, the (net) efficiency drops to a maximum of 1 to 2%, depending on the prevailing environmental conditions.
The total annual net primary production is estimated at 1.54 · 10 11 tons of plant biomass (dry matter).
One hectare of deciduous forest produces around 12 tons of organic matter in net primary production per year. This biomass corresponds to an energy content of 230 · 10 9 joules.
However, the actual effectiveness under field conditions is lower for a number of reasons, including sub-optimal carbon dioxide concentrations.
Dependence on abiotic factors
Photosynthesis depends on a number of abiotic factors that also influence one another. The law of the minimum applies here : Photosynthesis is restricted by the relatively scarce resource. To quantify photosynthesis, one can define the so-called photosynthesis rate. It is measured either as the amount of oxygen or glucose produced per unit of time. It can also be given as CO 2 uptake per unit of time.
Growth and yield in cultivated plants are increased by taking into account the factors mentioned below in greenhouse cultures.
carbon dioxide
Since carbon dioxide is fixed in photosynthesis, it depends on a sufficiently high CO 2 concentration. In today 's earth's atmosphere (2019) the CO 2 concentration is 0.041% by volume (% by volume). In 1 m³ of air there are 14 to 19 mmol CO 2 at room temperature .

The rate of photosynthesis of C 3 plants increases with increasing CO 2 concentration in the air (see figure on the right). Only when the CO 2 concentration is sufficiently high does it exceed the photosynthesis rate of the C 4 plants. Under atmospheric conditions (0.04% by volume) photosynthesis in C 3 plants is always inferior to that in C 4 plants and is suboptimal. If the CO 2 concentration drops too much, the carbon loss from respiration exceeds the carbon gain from photosynthesis. The point at which breathing and photosynthesis, i.e. CO 2 formation and fixation, are in balance, is the CO 2 compensation point Γ. In the case of C 3 plants, this point is 0,00 C3 = 0.005 to 0.010% by volume of CO 2 concentration; the rate of photosynthesis is saturated at 0.05 to 0.10% by volume. For C 4 plants, Γ C4 is 0.001% by volume. Thanks to their CO 2 pump, these plants can photosynthesize even with a very low CO 2 concentration.
The local CO 2 concentration in air layers close to the ground can be increased by fertilizing with compost . Here, microorganisms use the organic material oxidatively, so that u. a. CO 2 is released. In greenhouses , the CO 2 concentration is increased by fumigation and leads to an increased biomass yield through increased photosynthesis. However, the light intensity must not become a limiting factor (see next section).
Too high a CO 2 concentration (above 1% by volume) can damage many plants.
light
Photosynthesis is a light-powered process and the rate of photosynthesis depends primarily on the light intensity . Up to a species-specific light intensity, the photosynthesis rate increases with the light intensity. The leaves of a plant often follow the position of the sun and are as perpendicular as possible to the light in order to increase the illuminance . The position of the chloroplasts is also aligned for the highest possible rate of photosynthesis. In weak light, for example with heavy cloud cover, the broad side of the chloroplasts is exposed to light, while this is the narrow side under strong light. This orientation is mediated by the cytoskeleton .
In the case of C 3 plants, saturation occurs with increasing illuminance; a further increase in illuminance does not increase the rate of photosynthesis. This point is the light saturation point . The reason for this is the limiting CO 2 concentration in the air. At 0.03% by volume, this is suboptimal for C 3 plants (see section above). However, in comparison to C 3 plants, C 4 plants are not dependent on the atmospheric CO 2 concentration. Therefore, when the light intensity is increased - even in full sunlight - your photosynthesis rate does not experience saturation and is always light-limited. Furthermore, the light saturation occurs with different plants at different illuminance levels: " light plants " and " shadow plants ".

The so-called “ shadow plants ” are adapted to lower light intensities and the so- called “ sun plants ” or “light plants ” to higher light intensities . An analogous differentiation of the leaf shape can also occur within one and the same plant. In the case of the beech, for example, there are thick, small sun leaves , while the thin, large shadow leaves are close to the ground with lower light intensities. The palisade parenchyma is also more complex in the sun leaves, so that the strong solar radiation can be better exploited.
Sun plants (and sun leaves) such as cress only have a high rate of photosynthesis at high light intensities, the light saturation here is much higher than with shade plants (or shade leaves ). Shade plants, for example wood sorrel , can photosynthesize even at lower light intensities. However, the photosynthesis rate is lower with them than with solar plants, since the light saturation is reached at low illuminance levels (see also figure).
At low illuminance levels, photosynthesis takes place with very little efficiency, so that the carbon gain in the form of assimilates (and the production of oxygen) is less than the carbon (assimilate) loss (and oxygen consumption) in cellular respiration . The point at which photosynthesis and cell respiration are in balance is the light compensation point . This is different in different plants, highest in C 4 plants and lower in sun plants. Shade plants have the lowest light compensation point and as a result can still carry out net photosynthesis even at very low light intensities.
Excessive illuminance can lead to damage ( photo destruction ) and thus to a reduction in the rate of photosynthesis. This is the case, for example, with shade plants that are suddenly exposed to the blazing sun. Sunlight can also cause damage at low temperatures because of the reduced enzyme activity.
After it was recognized that the photosynthesis rate with a mixture of different light colors is higher than with irradiation with monochromatic light ( Emerson effect ), i.e. that there is mutual influence, it was proposed in 2009 that the quantum yield of additional monochromatic light be used to determine the photosynthetically active radiation to measure different wavelengths under white background lighting . This led to the realization that the photosynthetic quantum yield of green light is roughly the same as that of red light and greater than that of blue light. As early as 2004, practical tests led to faster plant growth and higher biomass yields after the addition of green light (500 to 600 nm). In addition, the alignment of the leaves (in the direction of the light source) with the help of green light can lead to a higher rate of photosynthesis due to photomorphogenesis , which results in more biomass.
Water supply and humidity
Although water is included in the photosynthesis equation, it is always available in sufficient quantities for the biochemical reaction. It is estimated that 1875 km³ of water are converted into photosynthesis each year. However, the CO is carried out 2 influx in the leaves through the stomata , which - depending on the humidity present open or closed - or water supply of the sheet. As a result, the air humidity and the water supply to the higher plants via the roots ( water stress , drought stress ) have an effect on photosynthesis: When it is dry, the stomata are closed by the guard cells to protect the plant from drying out. As a result, hardly any CO 2 gets into the leaf, so that it becomes a limiting factor. Due to their CO 2 enrichment, C 4 plants are not as severely affected as C 3 plants (see above). A special adaptation to lack of water is the Crassulacean acid metabolism in so-called CAM plants.
Artificial irrigation can increase the humidity and thus the rate of photosynthesis.
temperature
| Plant type | Minimum temperature | Temperature optimum | Temperature maximum |
| C 4 plant | 5 to 7 ° C | 35 to 45 ° C | 50 to 60 ° C |
| C 3 plant | −2 to 0 ° C | 20 to 30 ° C | 40 to 50 ° C |
| Sun plant | −2 to 0 ° C | 20 to 30 ° C | 40 to 50 ° C |
| Shade plant | −2 to 0 ° C | 10 to 20 ° C | 40 to 50 ° C |
| Evergreen tropical deciduous trees | 0 to 5 ° C | 25 to 30 ° C | 45 to 50 ° C |
| Deciduous trees of moderate latitudes | −3 to −1 ° C | 15 to 25 ° C | 40 to 45 ° C |
| Conifers | −5 to −3 ° C | 10 to 25 ° C | 35 to 40 ° C |
| weave | −15 to −10 ° C | 5 to 15 ° C | 20 to 30 ° C |
Photosynthesis is partly a question of biochemical reactions. Like every (bio) chemical reaction, this also depends on the temperature - in contrast to the photochemical processes. The optimal temperature for the enzymes (mainly for the light-independent part of photosynthesis) also determines the optimal temperature for a maximum rate of photosynthesis. This is because the electron acceptor of the light-dependent part of photosynthesis (NADP + ) is regenerated (oxidized) in the Calvin cycle and, if there is a deficiency in NADP + , photosynthesis is also restricted. Photosynthesis can only take place above a minimum temperature, for example, it is −1 ° C in frost-hardy plants. (see also the table below). The rate of photosynthesis increases with increasing temperature. According to van 't Hoff's RGT rule , the rate of reaction generally doubles to quadruple when the temperature is increased by 10 ° C. Photosynthesis eventually reaches an optimum temperature. For the plants in our latitudes, the optima are between 20 and 30 ° C. For thermophilic cyanobacteria, however, the optimum temperature is 70 ° C.
After this optimum has been reached, the photosynthetic output falls again due to the beginning denaturation of the proteins of the enzymes responsible for photosynthesis and finally comes to a complete standstill.
Chlorophyll content
Due to the high chlorophyll content in the cells, it never becomes a limiting factor in photosynthesis. Only certain variations of sun and shade plants can be observed. The latter have a higher chlorophyll content than sun plants and particularly large grana . The larger antennae of the shade plants also have a higher chlorophyll a / b ratio than sun plants. This closes the green gap a little better.
meaning
Under the current conditions of solar energy irradiation, about 440 billion tons of carbon dioxide are bound by plants each year, of which about 220 billion tons are released back into the atmosphere through plant respiration , the rest is bound as biomass or carried into the ground. Photosynthesis directly or indirectly drives all biogeochemical cycles in all existing ecosystems on earth. Even the lithotrophic communities at hydrothermal sources, which use inorganic compounds of geothermal origin as an energy source and are completely cut off from the light of the sun, are dependent on oxygen, the by-product of photosynthesis.
At present, terrestrial plants are responsible for around 50% of primary photosynthetic production . 30% are accounted for by algae and autotrophic protists, for example among the dinoflagellates , 20% by prokaryotes such as cyanobacteria .
The global CO 2 fixation takes place almost exclusively through the process of oxygenic photosynthesis, through plants and photosynthetic bacteria. Photosynthetic aerobic bacteria in the sea make up 2 to 5% of marine photosynthesis. The importance of anoxygenic photosynthesis for global CO 2 fixation is less than 1%. There are two reasons for this. On the one hand, phototrophic sulfur bacteria, which represent the dominant group among the anoxygenic phototrophic organisms in the ecosystems, only occur in high densities in some limnic and marine tidal zones. The ecosystems in question for these organisms also only contribute around 4% to global primary production. In lakes with phototrophic sulfur bacteria, the proportion of primary production through anoxygenic photosynthesis is around 29%. Therefore, based on current research, it is assumed that anoxygenic photosynthesis contributes less than 1% to global primary production. The second limiting factor for the contribution of anoxygenic photosynthesis to global primary production is the dependence of these organisms on reduced sulfur compounds . These compounds result from the anaerobic degradation of organic compounds to CO 2 with sulfate ; the so-called bacterial sulfate reduction . Since this organic carbon has previously been fixed by oxygenic photosynthesis, photosynthesis based on bacteriogenic sulfur compounds does not result in a net increase in organic compounds for the higher trophic levels in the food chain. For this reason, Norbert Pfennig introduced the term “secondary primary production” in 1978. Anoxygenic phototrophic organisms can therefore only compensate for the losses of organic compounds that arise during mineralization. The geothermal sulfur springs are an exception to this, as the reduced sulfur compounds come from abiotic sources.
In addition to the fixation of CO 2 , the formation of oxygen also plays an important role in oxygenic photosynthesis . On earth, elementary, molecular oxygen (dioxygen, O 2 ) is present in gaseous form in the earth's atmosphere and dissolved in water. About 99% of the oxygen comes from photosynthesis. Without oxygenic photosynthesis, aerobic organisms such as humans and animals could not live because they need it for breathing.
- All fossil raw materials and energy stores such as lignite , hard coal and crude oil are also by-products of photosynthesis.
- In the stratosphere , ozone (O 3 ) is formed from dioxygen (O 2 ) , which absorbs a large part of the UV radiation that is harmful to living beings . Only then has life on land become possible.
- The vegetation creates a more balanced climate through shading and evaporation .
evolution
Because of the importance of photosynthesis for life on earth, science was very early concerned with the origin and development of photosynthesis. To answer this question, data from different disciplines such as geology , biogeochemistry , comparative biochemistry and molecular evolution were collected. However, answering this question remains a scientific challenge and it has not yet been conclusively clarified. In some cases, it is even believed that the traces necessary to answer the question no longer exist, as photosynthesis originated very early in the development of life and the earth and its traces were lost over time.
What is certain, however, is that anoxygenic photosynthesis preceded oxygenic photosynthesis. Anoxygenic photosynthesis could have been established around 3.5 billion years ago ( Ga ). According to another estimate, photosynthesis with hydrogen (H 2 ) as a reducing agent was carried out before 3.8 Ga . Before 3.4 Ga, photosynthesis was carried out with H 2 S, before 3.0 Ga also with Fe 2+ as a reducing agent (by protocyanobacteria and proteobacteria).
Of great interest is the determination of the epoch in which oxygenic photosynthesis was carried out by protocyanobacteria . This is still controversially discussed in science, but the majority of the opinion is emerging that oxygenic photosynthesis must have already been well established when the almost anoxic atmosphere was enriched with oxygen ( Great Oxidation Event ) . This point in time of atmospheric oxygen enrichment is presumably between 2.3 and 2.4 Ga before the present. From this event, however, one cannot infer when oxygenic photosynthesis began. Because the first biochemically generated oxygen in all probability did not get into the atmosphere, but was used for the oxidation of dissolved substances (including Fe 2+ ).
In order to narrow down the time, various clues (markers) from three main directions are given: stromatolites , microfossils and molecules called biomarkers .
Stromatolites are laminated limestones made of alternating layers of biofilm (bio mat) and sediment deposits . Stromatolites can be proven by fossil finds from - 2.8 Ga. However, there are also indications of stromatolites up to 3.1–3.5 Ga old. In some of these fossil stromatolites, structures can be seen which were interpreted as the remains of thread-like bacteria that resemble the phototrophic cyanobacteria that can be detected in the recent stromatolites. But neither the biogenic origin of these microfossils nor their activity as oxygenic phototrophs nor the biogenic origin of most stromatolites is certain.
In addition to not entirely reliable phylogenetic findings on phototrophic microorganisms (see below), marker molecules are also analyzed. These are special hydrocarbons, the occurrence of redox-sensitive metals ( Mo , Re ) and the composition of specific, isotopic systems. Unique hydrocarbon marker for cyanobacteria Hopane , but will also steranes investigated. The isotopic composition of uranium - thorium - lead can be used to assess whether anoxic or oxic conditions existed: Under oxic conditions, only uranium forms soluble oxides and is therefore "more mobile".
The following schedule can be estimated from the data collected:
- before 3.8 Ga: possibly the first traces of local oxygen enrichment in the soil (U-Th-Pb measurements); However, these do not necessarily have to indicate the presence of the first oxygenic photosynthesis
- 3.2 Ga ago : first signs of oxygenic photosynthesis in today's Australia: thick, non- pyritic , kerogen- rich black slate
- before 2.72 Ga: Stromatolites in lake sediment layers indicate an established oxygenic photosynthesis
- before 2.5 Ga: Mo, Re markers indicate an O 2 surge
- before 2.45 Ga: numerous steranes and hopanes show that oxygenic photosynthesis is established
- before 2.3 Ga: oxygenic photosynthesis established beyond doubt, O 2 concentration in the atmosphere increased sharply
Nevertheless, the above schedule is also criticized and the point in time for the emergence of oxygenic photosynthesis at the time of the Makganyene Ice Age (before about 2.2 Ga). This is because, for example, hydrogen peroxide (H 2 O 2 ) collects in the ice and can later be released in larger quantities. H 2 O 2 and also O 2 are generated from water by abiotic, photochemical processes using UV light . It is also possible that hopanes are also formed by anoxygenic phototrophs.
Another method used earlier was to analyze the isotopic composition of carbon. This allows conclusions to be drawn as to whether CO 2 has been fixed biotically. The key enzyme of oxygenic photosynthesis, RubisCO, is decisive for this. During the assimilation of C from CO 2 during photosynthesis, the heavier but stable 13 C-carbon isotope is discriminated , while the lighter 12 C-carbon isotope is increasingly incorporated by the RuBisCO. As a result, carbon bound in organic substances is poorer at 13 C compared to carbon in inorganic substances . Measurements on organic and inorganic carbon compounds from 3.5–3.8 Ga old sediments gave a δ 13 C of −27 to +7 ‰ for the organic portion and +0.4 to +2.6 ‰ for the inorganic portion. Since these values coincide very well with today's measurements, this has long been regarded as an indication of an initial biogenic CO 2 fixation. However, whether this was a photosynthesis-driven CO 2 fixation cannot be deduced from the data, since chemolithotrophic CO 2 fixers also have similar δ 13 C values. This makes this method unsuitable for dating photosynthesis.
Photosynthetic systems
A comparison of the genome of five types of bacteria, each representing one of the five basic types of bacterial photosynthesis, showed that the components of the photosynthetic apparatus initially developed independently of one another in different bacteria and were put together by horizontal gene transfer . A comparison of the genes that these bacteria have in common with the genomes of other bacteria incapable of photosynthesis showed that most of the photosynthesis genes also occur in these bacteria. Whether the Chloroflexaceae ( green non-sulfur bacteria ) were the first organisms to become photoautotrophic through horizontal gene transfer is up for debate. A good candidate for a first photoautotrophy is the now extinct protocyanobacteria (syn. Procyanobacteria or pro-protocyanobacteria), hypothetical anoxygenic precursors of today's cyanobacteria. These could have passed on genes to Heliobacteria, Chloroflexaceae, purple bacteria and Chlorobiaceae by means of horizontal gene transfer.
Sequence data alone cannot be used to determine which type of bacteria was the first to photosynthesize. For this, data from other (independent) sources (see section above) must be used.
technology
- One attempt to make light usable in technical systems is, for example, the Grätzel cell . The aim is to produce organic solar cells with a high degree of efficiency for generating electricity. Here, like photosynthesis, the light energy is made usable by means of organic substances, but unlike photosynthesis, no substances are synthesized.
- Algae are cultivated in bioreactors ( algae reactors ). This allows CO 2 to be sequestered industrially and food and fuel to be produced.
See also
- Chemotrophy ( chemosynthesis )
literature
- Katharina Munk (Ed.): Pocket textbook Biology: Microbiology . Thieme Verlag, Stuttgart 2008, ISBN 978-3-13-144861-3 .
- Hans W. Heldt , Birgit Piechulla: Plant biochemistry . 4th edition. Spectrum Akademischer Verlag, Heidelberg 2008, ISBN 978-3-8274-1961-3 .
- Caroline Bowsher, Martin W. Steer, Alyson K. Tobin: Plant Biochemistry . Garland Pub, New York, NY 2008, ISBN 978-0-8153-4121-5 .
- David L. Nelson, Michael M. Cox: Lehninger Principles of Biochemistry. 5th edition. Freeman, New York, NY 2008, ISBN 978-0-7167-7108-1 .
- Georg Fuchs (Ed.): Thomas Eitinger, Erwin Schneider; Founded by Hans. G. Schlegel: General Microbiology. 8th edition. Thieme Verlag, Stuttgart 2007, ISBN 3-13-444608-1 .
- Neil A. Campbell: Biology . Spectrum textbook, 6th edition. Edited by J. Markl. Spektrum Verlag, Heidelberg, Berlin 2003, ISBN 3-8274-1352-4 .
- Donat-Peter Häder (Ed.): Photosynthesis . Georg Thieme Verlag, Stuttgart, New York 1999, ISBN 3-13-115021-1 .
- Andreas Bresinsky, Christian Körner, Joachim W. Kadereit, G. Neuhaus, Uwe Sonnewald: Strasburger - textbook of botany. 36th edition. Spectrum Akademischer Verlag, Heidelberg 2008, ISBN 978-3-8274-1455-7 .
- Ulrich Lüttge, Manfred Kluge, Gabriela Bauer: Botany . 5. completely revised Edition. Wiley-VCH, Weinheim 2005, ISBN 978-3-527-31179-8 .
- Peter H. Raven, Ray F. Evert, Susan E. Eichhorn: Biology of plants. 4th edition. Gruyter, Berlin, New York 2006, ISBN 978-3-11-018531-7 .
- Elmar Weiler, Lutz Nover, Wilhelm Nultsch: General and molecular botany . Thieme Verlag, Stuttgart 2008, ISBN 978-3-13-147661-6 .
- MF Hohmann-Marriott, RE Blankenship: Evolution of photosynthesis. In: Annu Rev Plant Biol . Vol. 62, 2011, pp. 515-548. PMID 21438681 ; doi: 10.1146 / annurev-arplant-042110-103811
Web links
- Botany online: photosynthesis
- Photosynthetica scientific journal in English
- Photosynthesis Research scientific journal in English
- Original introduction in the form of books (English)
- Photosynthesis: the mystery of the electron cycle solved
Individual evidence
- ↑ Annette Junker: Vitamin D protects. In: Deutscher Apotheker-Zeitung 2008, No. 31, p. 31.
- ↑ a b c d M. T. Madigan, JM Martinko: Brock microbiology . Munich, 2006: 613-614, ISBN 3-8273-7187-2 .
- ^ BM Griffin, J. Schott, B. Schink: Nitrite, an electron donor for anoxygenic photosynthesis. In: Science 316, 2007, p. 1870 doi: 10.1126 / science.1139478
- ↑ a b M. T. Madigan, JM Martinko: Brock microbiology . Munich, 2006: 621, ISBN 3-8273-7187-2 .
- ↑ MT Madigan, JM Martinko: Brock microbiology . Munich, 2006: 456, ISBN 3-8273-7187-2 .
- ↑ MT Madigan, JM Martinko: Brock microbiology . Munich, 2006: 444-447, ISBN 3-8273-7187-2 .
- ↑ MT Madigan, JM Martinko: Brock microbiology . Munich, 2006: 448-449, ISBN 3-8273-7187-2 .
- ↑ David L. Nelson, Michael M. Cox: Lehninger Principles of Biochemistry. 5th edition. Freeman, New York, NY 2008, ISBN 978-0-7167-7108-1 , p. 761.
- ↑ DA Bryant. et al . Candidatus Chloracidobacterium thermophilum: An aerobic phototrophic acidobacterium. In: Science , 317 ; 523-526 (2007).
- ^ Karl Mägdefrau: History of Botany . Gustav Fischer, Stuttgart 1973. p. 80.
- ^ Karl Mägdefrau: History of Botany . Gustav Fischer, Stuttgart 1973. pp. 84-86.
- ↑ Ilse Jahn (Ed.): History of Biology . 3rd edition, Nikol special edition, Hamburg 2004, p. 319f.
- ↑ Ilse Jahn (Ed.): History of Biology . 3rd edition, Nikol special edition, Hamburg 2004, pp. 515–518.
- ↑ Elmar Weiler, Lutz Nover, Wilhelm Nultsch: General and molecular botany . Thieme Verlag, Stuttgart 2008, ISBN 978-3-13-147661-6 , p. 261.
- ↑ Georg Fuchs (Ed.): Thomas Eitinger, Erwin Schneider; Founded by Hans. G. Schlegel: General Microbiology. 8th edition. Thieme Verlag, Stuttgart 2007, ISBN 3-13-444608-1 , p. 425.
- ↑ a b Barber, J. (2009): Photosynthetic energy conversion: natural and artificial. In: Chem Soc Rev . 38 (1); 185-196; PMID 19088973 ; doi: 10.1039 / b802262n
- ↑ Hans W. Heldt, Birgit Piechulla: Plant biochemistry . 4th edition. Spektrum Akademischer Verlag, Heidelberg 2008, ISBN 978-3-8274-1961-3 , p. 88.
- ↑ A. Guskov et al. (2009): Cyanobacterial photosystem II at 2.9-A resolution and the role of quinones, lipids, channels and chlorides. In: Nat Struct Mol Biol . 16 (3); 334-342; PMID 19219048 ; doi: 10.1038 / nsmb.1559
- ↑ Rappaport et al (2002): Kinetics and pathways of charge recombination in photosystem II. In: Biochemistry 41 (26), pp. 8518-8527; PMID 12081503
- ↑ Dau et al . (2004): The structure of the manganese complex of Photosystem II in its dark-stable S 1-state — EXAFS results in relation to recent crystallographic data. In: Phys Chem Chem Phys 6 (20) pp. 4781-4792
- ↑ Ferreira et al . (2004): Architecture of the photosynthetic oxygen-evolving center. In: Science 303 (5665), pp. 1831-1838; PMID 14764885
- ↑ JS Rieske, DH Maclennan, Coleman, R. (1964): Isolation and properties of an iron-protein from the (reduced coenzyme Q) -cytochrome C reductase complex of the respiratory chain. In: Biochemical and Biophysical Research Communications 15 (4); 338-344; doi: 10.1016 / 0006-291X (64) 90171-8
- ↑ Mitchell (1975): Proton motive redox mechanism of Cytochrome-b-c1 complex in respiratory chain - proton motive ubiquinone cycle. In: FEBS Lett 56 (1) pp. 1-6
- ↑ a b c Baniulis et al (2008): Structure-function of the cytochrome b6f complex. In: Photochem Photobiol 84 (6) pp. 1349-1358, PMID 19067956
- ^ Haehnel (1984): Photosynthetic Electron Transport in Higher Plants. In: Annu Rev Plant Biol 35 pp. 659-693.
- ↑ Hope (2000): Electron transfers amongst cytochrome f, plastocyanin and photosystem I: kinetics and mechanisms. In: Biochim Biophys Acta 1456 (1) pp. 5-26; PMID 10611452
- ↑ David L. Nelson, Michael M. Cox: Lehninger Principles of Biochemistry. 5th edition. Freeman, New York, NY 2008, ISBN 978-0-7167-7108-1 , p. 753.
- ↑ Sacksteder et al (2000): The proton to electron stoichiometry of steady-state photosynthesis in living plants: A proton-pumping Q cycle is continuously engaged. In: Proc Natl Acad Sci USA 97 (26) pp. 14283-14288; PMID 11121034
- ↑ Mitchell (1961): Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. In: Nature 191 (4784) pp. 144-148
- ↑ Seelert et al : Structural biology. Proton-powered turbine of a plant motor. In: Nature 405 (6785) pp. 418-419; PMID 10839529
- ^ Junge and Nelson (2005): Structural biology. Nature's rotary electromotors. In: Science 308 (5722) pp. 642-644; PMID 15860615
- ↑ A. Robertazzi, A. Galstyan, Knapp, EW. (2014): Reprint of PSII manganese cluster: protonation of W2, O5, O4 and His337 in the S1 state explored by combined quantum chemical and electrostatic energy computations. In: Biochim Biophys Acta . 1837 (9); 1389-1394; doi: 10.1016 / j.bbabio.2014.07.008 ; PMID 25065862
- ↑ a b c M. Suga et al. (2014): Native structure of photosystem II at 1.95 Å resolution viewed by femtosecond X-ray pulses. In: Nature PMID 25470056 ; doi: 10.1038 / nature13991
- ↑ a b c K. Sauer, J. Yano, Yachandra, VK. (2008): X-Ray spectroscopy of the photosynthetic oxygen-evolving complex. In: Coord Chem Rev . 252 (3-4); 318-335; PMID 19190720 ; PMC 2387253 (free full text)
- ↑ EM Sproviero et al. (2008): Quantum mechanics / molecular mechanics study of the catalytic cycle of water splitting in photosystem II. In: J Am Chem Soc . 130 (11); 3428-3442; PMID 18290643 ; doi: 10.1021 / ja076130q .
- ↑ H. Dobbek et al. (2001): Crystal structure of a carbon monoxide dehydrogenase reveals a [Ni-4Fe-5S] cluster. In: Science 293 (5533); 1281-1285; PMID 11509720 ; doi: 10.1126 / science.1061500 .
- ↑ Y. Pushkar et al. (2008): Structural changes in the Mn 4 Ca cluster and the mechanism of photosynthetic water splitting. In: Proc Natl Acad Sci USA 105 (6); 1879-1884; PMID 18250316 ; PMC 2542863 (free full text)
- ↑ Debus, RJ (2015): FTIR studies of metal ligands, networks of hydrogen bonds, and water molecules near the active site Mn4CaO5 cluster in Photosystem II. In: Biochim Biophys Acta 1847 (1); 19-34; PMID 25038513 ; doi: 10.1016 / j.bbabio.2014.07.007
- ↑ Barber, J. (2008): Photosynthetic generation of oxygen. In: Philos Trans R Soc Lond B Biol Sci . 363 (1504); 2665-2674; PMID 18468983 .
- ↑ a b Conlan, B. (2008): Designing photosystem II: molecular engineering of photo-catalytic proteins. In: Photosynth Res . 98 (1-3); 687-700; PMID 18777102 ; doi: 10.1007 / s11120-008-9355-5 .
- ↑ Camera is running! Researchers film Photosystem II at work Redaktion Pflanzenforschung.de; dated July 23, 2014, accessed October 28, 2014.
- ↑ B. Kok, B. Forbush, McGloin, M. (1970): Cooperation of charges in photosynthetic O 2 evolution-I. A linear four step mechanism. In: Photochem Photobiol . 11 (6); 457-475; PMID 5456273 ; doi: 10.1111 / j.1751-1097.1970.tb06017.x .
- ↑ Toshiharu Shikanai: Regulatory network of proton motive force: contribution of cyclic electron transport around photosystem I . In: Photosynthesis Research . tape 129 , no. 3 , September 1, 2016, p. 253–260 , doi : 10.1007 / s11120-016-0227-0 ( springer.com [accessed March 31, 2017]).
- ↑ Hans W. Heldt, Birgit Piechulla: Plant biochemistry . 4th edition. Spektrum Akademischer Verlag, Heidelberg 2008, ISBN 978-3-8274-1961-3 , pp. 103ff.
- ↑ Mehler, AH. (1951): Studies on reactions of illuminated chloroplasts: I. Mechanism of the reduction of oxygen and other hill reagents. In: Arch Biochem Biophys 33 (1); 65-77; PMID 14857775 ; doi: 10.1016 / 0003-9861 (51) 90082-3
- ↑ AH Mehler, Brown, AH. (1952): Studies on reactions of illuminated chloroplasts. III. Simultaneous photoproduction and consumption of oxygen studied with oxygen isotopes. In: Arch Biochem Biophys 38 (1); 365-370; PMID 12997112 ; doi: 10.1016 / 0003-9861 (52) 90042-8
- ↑ a b Asada, K. (2000): The water-water cycle as alternative photon and electron sinks. In: Philos Trans R Soc Lond B Biol Sci . 355 (1402); 1419-1431; PMID 11127996 ; PMC 1692883 (free full text, PDF).
- ↑ C. Hackenberg. et al . (2009): Photorespiratory 2-phosphoglycolate metabolism and photoreduction of O2 cooperate in high-light acclimation of Synechocystis sp. strain PCC 6803. In: Planta 230 (4); 625-637; PMID 19578872 ; PMC 2729987 (free full text, PDF).
- ^ Albertsson (2001): A quantitative model of the domain structure of the photosynthetic membrane. In: Trends Plant Sci 6 (8) pp. 349-58.
- ↑ A. Shapiguzov et al. (2010): The PPH1 phosphatase is specifically involved in LHCII dephosphorylation and state transitions in Arabidopsis. In: PNAS 9; 107 (10); 4782-4787; PMID 20176943 ; PDF (free full text access)
- ↑ Caroline Bowsher, Martin W. Steer, Alyson K. Tobin: Plant Biochemistry . Garland Pub, New York, NY 2008, ISBN 978-0-8153-4121-5 , p. 89.
- ↑ Hans W. Heldt, Birgit Piechulla: Plant biochemistry . 4th edition. Spektrum Akademischer Verlag, Heidelberg 2008, ISBN 978-3-8274-1961-3 , p. 110.
- ^ R. Scheuermann, K. Biehler, T. Stuhlfauth & HP Fock (1991): Simultaneous gas exchange and fluorescence measurements indicate differences in the response of sunflower, bean and maize to water stress . In: Photosynthesis Research 27 (3): 189-197, doi : 10.1007 / BF00035840 (currently not available) .
- ^ BM Griffin, J. Schott, B. Schink: Nitrite, an electron donor for anoxygenic photosynthesis. In: Science 316 (5833), 2007, p. 1870; PMID 17600210 ; doi: 10.1126 / science.1139478 .
- ↑ Georg Fuchs (Ed.): Thomas Eitinger, Erwin Schneider; Founded by Hans. G. Schlegel: General Microbiology. 8th edition. Thieme Verlag, Stuttgart 2007, ISBN 3-13-444608-1 , p. 432.
- ↑ Blankenship, RE, Madigan, MT, and Bauer, CE (2006). Anoxygenic Photosynthetic Bacteria (Springer Science & Business Media).
- ↑ Katharina Munk (ed.): Pocket textbook Biology: Microbiology . Thieme Verlag, Stuttgart 2008, ISBN 978-3-13-144861-3 , p. 341.
- ↑ David L. Nelson, Michael M. Cox: Lehninger Principles of Biochemistry. 5th edition. Freeman, New York, NY 2008, ISBN 978-0-7167-7108-1 , p. 751.
- ↑ Katharina Munk (ed.): Pocket textbook Biology: Microbiology . Thieme Verlag, Stuttgart 2008, ISBN 978-3-13-144861-3 , p. 343.
- ↑ a b Georg Fuchs (ed.): Thomas Eitinger, Erwin Schneider; Founded by Hans. G. Schlegel: General Microbiology. 8th edition. Thieme Verlag, Stuttgart 2007, ISBN 3-13-444608-1 , p. 434.
- ↑ M. Heinnickel, Golbeck, JH. (2007): Heliobacterial photosynthesis. In: Photosynth Res . 92 (1); 35-53; PMID 17457690 ; doi: 10.1007 / s11120-007-9162-4 .
- ↑ JT Beatty et al .: An obligately photosynthetic bacterial anaerobe from a deep-sea hydrothermal vent. In: Proc Natl Acad Sci USA Vol. 102, No. 26, 2005, pp. 9306-9310. PMID 15967984 ; PDF (full text access)
- ↑ Photosynthesis in the deep sea. (No longer available online.) Archived from the original on January 29, 2012 ; Retrieved January 8, 2011 .
- ↑ Georg Fuchs (Ed.): Thomas Eitinger, Erwin Schneider; Founded by Hans. G. Schlegel: General Microbiology. 8th edition. Thieme Verlag, Stuttgart 2007, ISBN 3-13-444608-1 , p. 411
- ↑ Linktext , Tang et al., 2010
- ↑ Blankenship, RE, Madigan, MT, and Bauer, CE (2006). Anoxygenic Photosynthetic Bacteria (Springer Science & Business Media)
- ^ Andreas Bresinsky, Christian Körner, Joachim W. Kadereit, G. Neuhaus, Uwe Sonnewald: Strasburger - Textbook of Botany. 36th edition. Spektrum Akademischer Verlag, Heidelberg 2008, ISBN 978-3-8274-1455-7 , p. 317.
- ↑ Elmar Weiler, Lutz Nover, Wilhelm Nultsch: General and molecular botany . Thieme Verlag, Stuttgart 2008, ISBN 978-3-13-147661-6 , p. 279.
- ↑ Katharina Munk (ed.): Basic studies in biology. Botany . Spektrum Akademischer Verlag, Heidelberg 2001, ISBN 3-8274-0909-8 , pp. 6-6.
- ↑ Munk, Katharina (ed.): Basic studies in biology. Botany . Spektrum Akademischer Verlag, Heidelberg 2001, ISBN 3-8274-0909-8 , pp. 5-27.
- ^ A b Hermann Linder: Biology . Schroedel; 21., rework. Edition 1998, ISBN 3-507-10580-2 , p. 43.
- ^ A b Wilhelm Nultsch: General botany . Georg Thieme Verlag; 10th, revised edition 1996, ISBN 3-13-383310-3 , pp. 309-311.
- ↑ T. Stuhlfauth, HP Fock (1990): Effect of whole season CO 2 enrichment on the cultivation of a medicinal plant, Digitalis lanata. In: J Agronomy & Crop Science 164 (3), 168-173; doi: 10.1111 / j.1439-037X.1990.tb00803.x
- ↑ a b c Ulrich Lüttge , Manfred Kluge, Gabriela Bauer: Botany . 5. completely revised Edition. Wiley-VCH, Weinheim 2005, ISBN 978-3-527-31179-8 , pp. 472f.
- ↑ a b c Hermann Linder: Biology . Schroedel; 21., rework. Edition 1998, ISBN 3-507-10580-2 , pp. 48-49.
- ↑ T. Stuhlfauth and HP Fock, Effect of whole season CO 2 enrichment on the cultivation of a medicinal plant, Digitalis lanata, J. Agronomy & Science 164, 168-173 (1990) doi: 10.1111 / j.1439-037X.1990 .tb00803.x
- ↑ Ulrich Lüttge, Manfred Kluge and Gabriela Bauer. Botany . Wiley-VCH; 5. completely revised Edition 2005, ISBN 978-3-527-31179-8 .
- ↑ Ulrich Lüttge and Manfred Kluge: Botany - The introductory biology of plants . Wiley-VCH Verlag GmbH & Co. KGaA; 6th updated edition 2012, ISBN 978-3-527-33192-5 , p. 498.
- ^ A b Andreas Bresinsky, Christian Körner, Joachim W. Kadereit, G. Neuhaus, Uwe Sonnewald: Strasburger - Textbook of Botany. 36th edition. Spektrum Akademischer Verlag, Heidelberg 2008, ISBN 978-3-8274-1455-7 , p. 315.
- ^ Hermann Linder: Biology . 21., rework. Edition. Schroedel Verlag, 1998, ISBN 3-507-10580-2 , p. 45.
- ^ Turrell (1934): The area of the internal exposed surface of dicotyledon leaves. In: American Journal of Botany 23 (4), 255-264, doi: 10.1002 / j.1537-2197.1936.tb08982.x .
- ↑ Ichiro Terashima, Takashi Fujita, Takeshi Inoue, Wah bald Chow, Riichi Oguchi: "Green Light Drives Leaf Photosynthesis More Efficiently than Red Light in Strong White Light: Revisiting the Enigmatic Question of Why Leaves are Green?" ; Physiology of Plants and Cells, Volume 50, Issue 4, April 1, 2009, published: February 25, 2009, pages 684-697, doi: 10.1093 / pcp / pcp034
- ↑ HH Kim, GD Goins, RM Wheeler, JC Sager: Green light supplementation for improved lettuce growth under red and blue light emitting diodes. ; HortScience, 2004, December; 39 (7): 1617-1622, PMID 15770792 .
- ↑ SW Hogewoning, G. Trouwborst, E. Meinen, W. van Ieperen, (2012): "Finding the Optimal Grow Light Spectrum for Greenhouse Crops" , Acta Hortic. 956, 357-363, doi: 10.17660 / ActaHortic.2012.956.41 .
- ^ Ulrich Lüttge, Manfred Kluge, Gabriela Bauer: Botany . 5. completely revised Edition. Wiley-VCH, Weinheim 2005, ISBN 978-3-527-31179-8 , p. 318.
- ↑ B. Steuer, T. Stuhlfauth and HP Fock: The efficiency of water use in water stressed plants is increased due to ABA induced stomatal closure, Photosynthesis Research 18, 1988, pp. 327–336 doi: 10.1007 / BF00034837
- ^ Ulrich Lüttge, Manfred Kluge, Gabriela Bauer: Botany . 5. completely revised Edition. Wiley-VCH, Weinheim 2005, ISBN 978-3-527-31179-8 , p. 472.
- ^ Hermann Linder: Biology . 21., rework. Edition. Schroedel Verlag, 1998, ISBN 3-507-10580-2 , pp. 48-49.
- ^ US Department of Energy : Carbon Cycling and Biosequestration. Integrating Biology and Climate Through Systems Science. Report from the March 2008 workshop. 2008. PDF (16 MB).
- ↑ Jane Reece & al .: Campbell Biology . 10th edition, Pearson, Hallbergmoos 2016, p. 795.
- ↑ ZS Kolber, CL Van Dover, RA Niederman, PG Falkowski: Bacterial photosynthesis in surface waters of the open ocean. In: Nature. Volume 407, Number 6801, September 2000, pp. 177-179, doi: 10.1038 / 35025044 . PMID 11001053 .
- ↑ Volker Storch, Ulrich Welsch, Michael Wink: Evolutionary Biology. Springer, 2013, ISBN 978-3-642-32836-7 , p. 93.
- ↑ EG Nisbet, Nisbet, RE. (2008): Methane, oxygen, photosynthesis, rubisco and the regulation of the air through time. In: Philos Trans R Soc Lond B Biol Sci . 363 (1504); 2745-2754; PMID 18487133 .
- ↑ a b c Olson, JM. (2006): Photosynthesis in the Archean era. In: Photosynth Res . 88 (2); 109-117; PMID 16453059 ; doi: 10.1007 / s11120-006-9040-5 .
- ↑ Andrew D. Czaja et al. a .: Biological Fe oxidation controlled deposition of banded iron formation in the ca.3770 Ma Isua Supracrustal Belt (West Greenland). In: Earth and Planetary Science Letters. Volume 363, 2013, pp. 192-203, doi: 10.1016 / j.epsl.2012.12.025
- ↑ a b c d Buick, R. (2008): When did oxygenic photosynthesis evolve? In: Philos Trans R Soc Lond B Biol Sci . 363 (1504); 2731-2743; PMID 18468984 .
- ^ R. Buick: Life in the Archean. In: Dereck Briggs, Peter R. Crowther: Paleobiology II . Wiley-Blackwell, Oxford 2001, ISBN 978-0-632-05149-6 , pp. 13-21.
- ↑ JL Kirschvink, Kopp, RE. (2008): Palaeoproterozoic ice houses and the evolution of oxygen-mediating enzymes: the case for a late origin of photosystem II. In: Philos Trans R Soc Lond B Biol Sci . 363 (1504); 2755-2765; PMID 18487128 .
- ↑ Schidlowski, M. et al . (1983): Isotopic inferences of ancient biochemistries: Carbon, sulfur, hydrogen, and nitrogen. In: JW Schopf (Ed.): Earth's Earliest Biosphere: Its Origin and Evolution Prince-ton, New Jersey, Princeton University Press, ISBN 978-0-691-02375-5 .
- ↑ J. Raymond, O. Zhaxybayeva, JP Gogarten, SY Gerdes, Blankenship RE (2002): Whole-genome analysis of photosynthetic prokaryotes. In: Science 298 (5598); 1616-1620; PMID 12446909 ; doi: 10.1126 / science.1075558 .
- ↑ a b A. Y. Mulkidjanian, EV Koonin, KS Makarova, SL Mekhedov, A. Sorokin, YI Wolf, A. Dufresne, F. Partensky, H. Burd, D. Kaznadzey, R. Haselkorn, Galperin MY (2006): The cyanobacterial genome core and the origin of photosynthesis. In: Proc Natl Acad Sci USA 103 (35); 13126-13131; PMID 16924101 ; PMC 1551899 (free full text)
- ↑ Pennisi, E. (2002): Evolutionary biology. Bacteria shared photosynthesis genes. In: Science 298 (5598); 1538-1539; PMID 12446882 ; doi: 10.1126 / science.298.5598.1538b .
- ↑ Energy sources with a future ( DFG )







![{\ mathrm {12 \ H_ {2} O \ {\ xrightarrow {h \ nu}} \ 24 \ [H] +6 \ O_ {2}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/8164717f489d1128fdf68c58053651b3ace1f47f)
![{\ mathrm {6 \ CO_ {2} +24 \ [H] \ longrightarrow C_ {6} H _ {{12}} O_ {6} +6 \ H_ {2} O}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/ab734440d539794119fcae6e343b784c286e6de5)