3-hydroxypropionate cycle
The 3-hydroxypropionate cycle , also 3-hydroxypropionate cycle or 3-hydroxypropionate / malyl-CoA cycle , is a metabolic pathway through which certain microorganisms can fix carbon dioxide (CO 2 ) . Here, two molecules of CO 2 in the form of bicarbonate (HCO 3 - ) are built up into one molecule of glyoxylate with the consumption of ATP and NADPH . The resulting glyoxylate is then converted to pyruvate in a subsequent cycle with the fixation of another molecule of bicarbonate . The 3-hydroxypropionate cycle was first discovered in 1986 in the phototrophic bacterium Chloroflexus aurantiacus .
Occurrence
The 3-hydroxypropionate cycle has so far only been detected in the microaerophilic green non-sulfur bacterium Chloroflexus aurantiacus . Although closely related bacteria ( Chloroflexus aggregans , Roseiflexus spp.) Have the genes for this metabolic pathway, autotrophic growth has not yet been detected in them. Another relative, Oscillochloris sp., Uses the Calvin cycle and not the 3-hydroxypropionate cycle for CO 2 fixation.
biochemistry
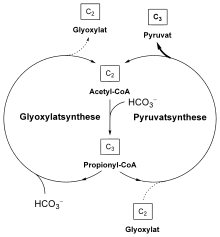
The 3-hydroxypropionate cycle consists of two interconnected cycles. In the first cycle, glyoxylate is made up of two molecules of bicarbonate. The glyoxylate is then converted to pyruvate in a second cycle ; another molecule of bicarbonate is used for regeneration in the second cycle. So three molecules of bicarbonate are built up into one molecule of pyruvate.
Formation of glyoxylate
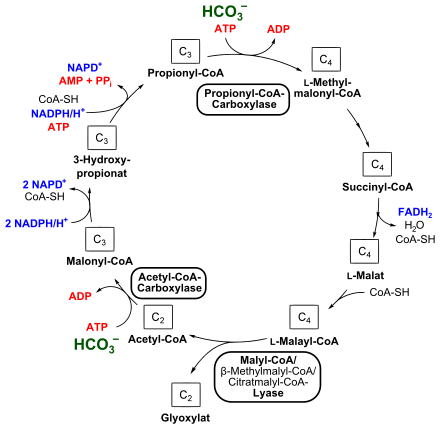
Starting with acetyl-CoA , the cycle starts with the condensation of one molecule of bicarbonate, which also consumes one molecule of ATP. This reaction is catalyzed by a biotin- containing acetyl-CoA carboxylase , resulting in malonyl-CoA. This is reduced to 3-hydroxypropionate by a malonyl-CoA reductase with NADPH consumption. 3-Hydroxypropionate is the eponymous component of this cycle. Propionyl-CoA synthase catalyzes the formation of propionyl-CoA; in this step, NADPH is again required as a reducing agent .
The second step of bicarbonate assimilation takes place in the conversion of propionyl-CoA to methylmalonyl-CoA with consumption of ATP, which enables propionyl-CoA carboxylase. Methylmalonyl-CoA is rearranged via succinyl-CoA and L - malate to L- malyl-CoA. An epimerase , a mutase , a transferase , a dehydrogenase and a fumarase (fumarate hydratase) are involved in this reaction sequence, whereby FADH 2 is also formed.
A malyl-CoA lyase, an enzymatic part of the trifunctional malyl-CoA / β-methylmalyl-CoA / citrate malyl-CoA lyase (see also below), finally splits L- malyl-CoA into glyoxylate and acetyl-CoA, which causes the circulation closes again.
The balance for the formation of glyoxylate is:
Formation of pyruvate
In the second cycle that follows, the glyoxylate built up is converted to pyruvate. One molecule of glyoxylate and propionyl-CoA each condense to form β-methylmalyl-CoA, which is catalyzed by the β-methylmalyl-CoA lyase (malyl-CoA / β-methylmalyl-CoA / citrate malyl-CoA lyase) involved in the first cycle . β-methylmalyl-CoA is converted to mesaconyl-C1-CoA by a mesaconyl-CoA hydratase. From a chemical point of view, mesaconyl-C1-CoA corresponds to 2-methylfumaryl-CoA. The further sequence of reactions was recently deciphered in C. aurantiacus . Mesaconyl-C1-CoA is converted to mesaconyl-C4-CoA. This intramolecular transesterification of coenzyme A is catalyzed by a transferase. Mesaconyl-C4-CoA is then converted to (3 S ) -citrate malyl-CoA, which catalyzes a hydratase. This is finally split into acetyl CoA and pyruvate - by a citrate malyl CoA lyase, the enzymatic part of the malyl CoA / β-methylmalyl CoA / citrate malyl CoA lyase. Acetyl-CoA is built up to propionyl-CoA as in the first part of the 3-hydroxypropionate cycle. Pyruvate is then finally metabolized further via a triose phosphate.
The balance for the formation of pyruvate from glyoxylate is:
The overall balance for the formation of a pyruvate molecule is as follows:
Biological importance
The fixation of three molecules of bicarbonate to pyruvate is a very energy-intensive process. Building up one molecule of glyceraldehyde-3-phosphate costs 10 equivalents of ATP (the AMP formed counts twice). However, the process can also take place under aerobic or microaerophilic conditions, since none of the enzymes involved are per se sensitive to oxygen. In addition to the fixation of bicarbonate, intermediates of the cycle can also be assimilated ( acetic acid , propionic acid , C 4 - dicarboxylic acids ). These are excreted as fermentation products by associated microorganisms.
A special feature of the cycle is that bi- and trifunctional enzymes are involved. Malonyl-CoA reductase catalyzes two reaction steps, malyl-CoA / β-methylmalyl-CoA / citrate malyl-CoA lyase and propionyl-CoA synthase can catalyze three reactions. The entire cycle takes place in 19 reactions, but only 13 enzymes are involved in the reactions.
In a similar form, the 3-hydroxypropionate / 4-hydroxybutyrate cycle , the fixation of bicarbonate in thermo- acidophilic archaea of the genera Metallosphaera , Acidianus and Sulfolobus has been demonstrated.
The use of bicarbonate instead of carbon dioxide can be explained by the fact that C. aurantiacus grows in waters with a slightly alkaline environment. Under these conditions the concentration of bicarbonate is higher than that of carbon dioxide.
Individual evidence
- ↑ Hans Günther Schlegel, Georg Fuchs (Ed.): General microbiology . 9th edition. Thieme, Stuttgart 2014, p. 313. ISBN 978-3-13-444609-8
- ↑ a b Thauer, RK. (2007): Microbiology. A fifth pathway of carbon fixation. In: Science 318 (5857); 1782-1783; PMID 18079388 ; doi: 10.1126 / science.1152209 .
- ↑ Holo, H. and Sirevåg, R. (1986): Autotrophic growth and CO 2 fixation of Chloroflexus aurantiacus . In: Arch. Microbiol. 145 (2); 173-180; doi: 10.1007 / BF00446776 .
- ↑ Klatt, CG. et al . (2007): Comparative genomics provides evidence for the 3-hydroxypropionate autotrophic pathway in filamentous anoxygenic phototrophic bacteria and in hot spring microbial mats . In: Environ Microbiol . 9 (8); 2067-2078; PMID 17635550 ; doi: 10.1111 / j.1462-2920.2007.01323.x .
- ↑ Ivanovsky, RN. et al . (1999): Evidence for the presence of the reductive pentose phosphate cycle in a filamentous anoxygenic photosynthetic bacterium, Oscillochloris trichoides strain DG-6 . In: Microbiology 145 (Pt 7); 1743-1748; PMID 10439413 .
- ↑ a b Zarzycki, J. et al. (2008): Mesaconyl-coenzyme A hydratase, a new enzyme of two central carbon metabolic pathways in bacteria. In: J Bacteriol. 190 (4); 1366-1374; PMID 18065535 ; PMC 2238226 (free full text, PDF).
- ↑ Zarzycki, J. et al . (2009): Identifying the missing steps of the autotrophic 3-hydroxypropionate CO2 fixation cycle in Chloroflexus aurantiacus . In: PNAS USA 106 (50); 21317-21322; PMID 19955419
- ↑ Georg Fuchs (Ed.), Hans. G. Schlegel (Author): General Microbiology. Thieme Verlag Stuttgart; 8th edition 2007; ISBN 3-13-444608-1 ; P. 245.
- ↑ G. Fuchs (Ed.): Mikrobiologie , p. 249.
- ^ Berg, IA. et al. (2007): A 3-hydroxypropionate / 4-hydroxybutyrate autotrophic carbon dioxide assimilation pathway in Archaea . In: Science 318 (5857); 1782-1786; PMID 18079405 ; doi: 10.1126 / science.1149976 .
- ↑ Katharina Munk (ed.): Pocket textbook Biology: Microbiology . Thieme Verlag Stuttgart 2008; ISBN 978-3-13-144861-3 ; P. 412.
Web links
- Overview with enzyme names (on MetaCyc)