Reductive citric acid cycle
The reductive citric acid cycle (also known as the reductive citric acid cycle, reductive citric acid cycle, reductive tricarboxylic acid cycle or reductive Krebs cycle) is a cyclic reaction path in which carbon dioxide (CO 2 ) fixation ( carbon dioxide assimilation ) takes place through retrograde steps of the citric acid cycle . By reversing the steps of the citric acid cycle, it is also known as the reverse citric acid cycle . The cycle was discovered in 1966 through the work of Mike C. Evans, Bob B. Buchanan and Daniel I. Arnon.
Occurrence
The reductive citric acid cycle has been demonstrated in various microaerobic and obligate anaerobic microorganisms. It was identified in green sulfur bacteria as well as in green non-sulfur bacteria . It was originally discovered in 1966 in the green sulfur bacterium Chlorobium limicola . It is assumed that the deeply branching thermophilic bacterium Aquifex aeolicus also fixes CO 2 via the reductive citric acid cycle .
It was previously suggested that archaea ( Thermoproteus neutrophilus ) can fix carbon dioxide via this metabolic pathway. However, this has been refuted by more recent research results.
biochemistry
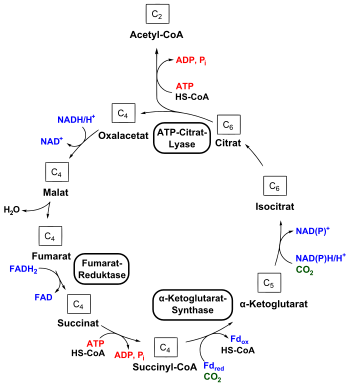
The reductive citric acid cycle is the reverse of the (oxidative) citric acid cycle. Most of the enzymes of the citric acid cycle are also used in this metabolic pathway - in the opposite direction. In the oxidative citric acid cycle, however, there are three irreversible steps that are circumvented in the reductive citric acid cycle by three special enzymes:
1.) The reduction of fumarate to succinate is catalyzed by a fumarate reductase . It replaces the succinate dehydrogenase in the ox. Citric acid cycle.
2.) A molecule of CO 2 condenses on succinyl-CoA with the consumption of reduced ferredoxin . This reductive carboxylation is catalyzed by the α-ketoglutarate synthase . This bypasses the decarboxylation mediated by α-ketoglutarate dehydrogenase in the ox. Citric acid cycle.
3.) In the ox. Citrate cycle condenses acetyl-CoA with oxaloacetate , which catalyzes the citrate synthase . In the reductive citric acid cycle, this reaction is reversed by an ATP citrate lyase , whereby citrate is split into acetyl CoA and oxaloacetate. This closes the circle. ATP is required for this reaction .
In the overall balance, eight reduction equivalents (in the form of NAD (P) H and red.ferredoxin) and two molecules of ATP are required to fix two molecules of CO 2 to acetyl-CoA :
For the reversal of the citric acid cycle, an additional molecule of ATP is required, as eight reduction equivalents and one molecule of GTP are released in the oxidative citric acid cycle :
Biological importance
The reductive citric acid cycle enables the above-mentioned microorganisms to fix CO 2 . However, acetyl-CoA is not assimilated via the glyoxylate cycle , but converted to pyruvate by a ferredoxin-dependent pyruvate synthase , a third CO 2 molecule is fixed in the process:
Pyruvate can finally be metabolized to hexoses in the course of gluconeogenesis and introduced into the building metabolism.
The cycle consumes significantly less ATP compared to the Calvin cycle: based on the formation of a glyceraldehyde-3-phosphate , rTCA consumes 5 ATP, whereas organisms with the Calvin cycle require 9 to 13 ATP. In general, because of the oxygen sensitivity of the enzymes involved, it can only take place under anaerobic or (micro) aerobic conditions. B. Hydrogenobacter thermophilus TK-6.
literature
- Georg Fuchs (ed.), Hans. G. Schlegel (Author): General Microbiology . Thieme Verlag Stuttgart, 8th edition 2007, ISBN 3-13-444608-1 , p. 247f.
- Katharina Munk (Ed.): Pocket textbook Biology: Microbiology . Thieme Verlag Stuttgart 2008, ISBN 978-3-13-144861-3 , p. 410f.
- Michael T. Madigan, John M. Martinko, Jack Parker, and Thomas D. Brock: Microbiology . Spektrum Akademischer Verlag, ISBN 3-8274-0566-1 , pp. 659f.
- Bob B. Buchanan et al .: The Arnon-Buchanan cycle: a retrospective, 1966-2016 . In: Photosynthesis Research . tape 134 , no. 2 , November 2017, p. 117-131 , doi : 10.1007 / s11120-017-0429-0 , PMID 29019085 .
Individual evidence
- ↑ Evans, MC. et al. (1966): A new ferredoxin-dependent carbon reduction cycle in a photosynthetic bacterium . In: PNAS 55 (4); 928-934; PMID 5219700 , PMC 224252 (free full text)
- ↑ Michael T. Madigan, John M. Martinko, Jack Parker and Thomas D. Brock: Microbiology . Spectrum Academic Publishing House; ISBN 3-8274-0566-1 ; P. 659
- ↑ Guiral M, Aubert C, Giudici-Orticoni MT: Hydrogen metabolism in the hyperthermophilic bacterium Aquifex aeolicus . In: Biochem. Soc. Trans. . 33, No. Pt 1, February 2005, pp. 22-4. doi : 10.1042 / BST0330022 . PMID 15667254 .
- ↑ Strauss, G. et al . (1992): 13C-NMR study of autotrophic CO2 fixation pathways in the sulfur-reducing Archaebacterium Thermoproteus neutrophilus and in the phototrophic Eubacterium Chloroflexus aurantiacus . In: Eur J Biochem . 205 (2); 853-866; PMID 1572376 .
- ^ WH Ramos-Vera, IA Berg, G. Fuchs: Autotrophic carbon dioxide assimilation in Thermoproteales revisited. In: Journal of bacteriology. Volume 191, Number 13, July 2009, pp. 4286-4297, doi : 10.1128 / JB.00145-09 , PMID 19411323 , PMC 2698501 (free full text).
- ↑ Arren Bar-Even, Elad Noor, Nathan E. Lewis, Ron Milo: Design and analysis of synthetic carbon fixation pathways . In: Proceedings of the National Academy of Sciences . 107, No. 19, November 5, 2010, ISSN 0027-8424 , pp. 8889-8894. doi : 10.1073 / pnas.0907176107 . PMID 20410460 . Retrieved October 30, 2013.
- ^ Berg, IA. et al . (2010): Study of the distribution of autotrophic CO2 fixation cycles in Crenarchaeota . In: Microbiology 156 (Pt 1); 256-269; PMID 19850614 ; doi : 10.1099 / mic.0.034298-0
![\ mathrm {2 \ CO_2 + 8 \ [H] + 2 \ ATP \ longrightarrow Acetyl \ text {-} CoA + 2 \ ADP + H_2O}](https://wikimedia.org/api/rest_v1/media/math/render/svg/ce67afbfd14ddd80e3ff741b5e600326c328979f)
![\ mathrm {Acetyl \ text {-} CoA + GDP + H_2O \ xrightarrow {Citrate cycle} 2 \ CO_2 + 8 \ [H] + GTP}](https://wikimedia.org/api/rest_v1/media/math/render/svg/103dfe4f520c56104f4582debda4998362c34f01)
