Plastocyanin
| Plastocyanin ( Spinacia oleracea ) | ||
|---|---|---|

|
||
| Ribbon model of the plastocyanin from spinach , according to PDB 1AG6 | ||
|
Available structure data : 1B3I , 1BAW , 2Q5B , 3BQV , 3PCY , 4PCY , 5PCY , 6PCY , 7PCY , 9PCY |
||
| Mass / length primary structure | 99 amino acids, 10.4 kDa (spinach) | |
| Cofactor | Copper ion | |
| Isoforms | PET1 , PET2 in A. thaliana | |
| Identifier | ||
| Gene name (s) | PETE | |
| External IDs | ||
| Occurrence | ||
| Parent taxon | Plants, cyanobacteria | |
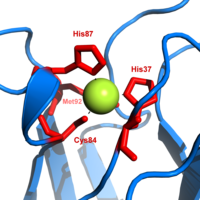
Plastocyanin , sometimes called plastocyanin , is a small copper protein found in algae , green plants, and some cyanobacteria that plays an important role in photosynthesis. It transports electrons from the cytochrome b 6 f complex of photosystem I . In eukaryotes, plastocyanin is localized in the lumen of the thylakoids .
Plastocyanin is one of the blue copper proteins found in plants, archaea and bacteria.
structure
In spinach and most other organisms, plastocyanin weighs 10.5 kDa in its mature form and is made up of 99 amino acids. As a cofactor, it binds a copper atom coordinatively, which changes between the oxidation states Cu II and Cu I by accepting electrons . The protein can only accept and release one electron. The tertiary structure of the protein is a β-barrel .
function
The light reaction of photosynthesis is a sequence of electron transfers that takes place in plants in the thylakoids of the chloroplasts. The protein complex Photosystem II uses light energy to extract electrons from water. The electrons are first transferred to the small molecule plastoquinone , and then via the cytochrome b 6 f complex to plastocyanin and finally via photosystem I to NADP + . Plastocyanin is a soluble electron carrier that diffuses in the lumen of the thylakoid.
The electron is transferred directly to plastocyanin from cytochrome f , a subunit of the cytochrome b 6 f complex. In some cyanobacteria and algae, plastocyanin is replaced by the small, iron-containing protein cytochrome c 6 . Plastocyanin and cytochrome c 6 interact directly with photosystem I and reduce its photooxidized reaction center P700 + .
Expression and Topogenesis
In plants and green algae, plastocyanin is encoded in the core by the gene petE , the cytosolically translated product of which is a prepro- apoprotein with an N-terminal transit peptide , which is split off by a peptidase after being imported into the chloroplast . This intermediate form is then transported via the SecA transport route into the thylakoid lumen, where it binds the copper atom.
Regulation of petE expression
The petE gene expression is partly regulated by the cellular sugar status ( sugar sensing ), so the expression is inhibited by the accumulation of sugars. Furthermore, a reduction in the plastoquinone pool and the thioredoxin system, as well as the quality of the irradiated light (or a change from preferentially photosystem-II-stimulating light to photosystem-I-stimulating light) also repress petE expression.
In addition, the petE expression is suppressed by the presence of reactive oxygen species . This requires retrograde signaling pathways that regulate the gene expression of nucleus-encoded genes as a function of plastid signals. In the studies mentioned, however, only the petE gene expression and not the accumulation of the protein were analyzed, which do not necessarily have to match.
Interaction with the photosynthetic complexes
Plastocyanin forms a solid transition complex with cytochrome f , which enables very fast electron transfer within 35 to 350 µs.
Arginines and lysines in the region between the large and small domains of cytochrome f bind plastocyanin possibly via electrostatic forces. In higher plants, the reduced plastocyanin interacts directly with photosystem I and forms a transition complex with it. The complex then undergoes a conformational change and an electron is passed on to P700 + . The electron transfer from plastocyanin to P700 is fast with a duration of 10 to 20 µs, since reduced plastocyanin is bound more strongly than oxidized plastocyanin. In the crystal structures of photosystem I, a flat region can be seen on the lumen side of the photosystem, which is probably the binding site for plastocyanin. Luminal loops of PsaA and PsaB contribute to the efficient binding of plastocyanin. In plants, additional amino acids at the N-terminus of PsaF achieve a much stronger plastocyanin bond than in most cyanobacteria - in these, plastocyanin interacts by collision and not specifically with photosystem I; the measured transfer speeds are up to two orders of magnitude lower here. However, in cyanobacteria a solid transition complex forms between photosystem I and the alternative electron carrier cytochrome c 6 .
literature
- Hans-Walter Heldt and Fiona Heldt (1999): Plant Biochemistry . Spectrum Academic Publishing House, Heidelberg.
- K. Sigfridsson (1998): Plastocyanin, an electron transfer protein . In: Photosynth Res 57 (1) pp. 1-28; doi : 10.1023 / A: 1006067631076
- GS Singhal, G. Renger and SK Sopory (1999): Concepts in Photobiology: Photosynthesis and Photomorphogenesis . Jumper.
Individual evidence
- ↑ a b Y. Xue, M. Okvist, O. Hansson, S. Young: Crystal structure of spinach plastocyanin at 1.7 A resolution. In: Protein science: a publication of the Protein Society. Volume 7, number 10, October 1998, pp. 2099-2105, doi : 10.1002 / pro.5560071006 , PMID 9792096 , PMC 2143848 (free full text).
- ↑ Peter Schopfer and Axel Brennicke: Plant Physiology . Elsevier, Munich 2006. ISBN 978-3-8274-1561-5 , p. 189
- ↑ UniProt P00289
- ↑ Interpro: Plastocyanin
- ↑ Swiss Institute of Bioinformatics (SIB): PROSITE documentation PDOC00174. Type 1 (blue) copper proteins. Retrieved August 12, 2011 .
- ↑ Sato K., Kohzuma T., Dennison C. (2003): Active-site structure and electron-transfer reactivity of plastocyanins . In: JACS vol. 125 (8) pp. 2101-2112; PMID 12590538
- ↑ a b Jordan P., Fromme P., Witt HT, Klukas O., Saenger W., Krauss N. (2001): Three-dimensional structure of cyanobacterial photosystem I at 2.5 Å resolution . In: Nature 411 (6840) pp. 909-917; PMID 11418848
- ↑ a b Ben-Shem A. Frolov F., Nelson N. (2003): Crystal structure of plant photosystem I . Nature 426 (6967) pp. 630-635; PMID 14668855
- ^ Rother C., Jansen T., Tyagi A., Tittgen J., Herrmann RG (1986): Plastocyanin is encoded by an uninterrupted nuclear gene in spinach . In: Curr Genet 11 (3) pp. 171-176; PMID 2834087
- ↑ Li HH, Quinn J., Culler D., Girard-Bascou J., Merchant S. (1996): Molecular genetic analysis of plastocyanin biosynthesis in Chlamydomonas reinhardtii . In: J Biol Chem 271 (49) pp. 31283-9
- ↑ Dijkwel PP, Kock P., Bezemer R., Weisbeek PJ, Smeekens SC (1996): Sucrose Represses the Developmentally Controlled Transient Activation of the Plastocyanin Gene in Arabidopsis thaliana Seedlings . In: Plant Physiol 110 (2) pp. 455-463; PMID 12226197
- ↑ Oswald O., Martin T., Dominy PJ, Graham IA (2001): Plastid redox state and sugars: interactive regulators of nuclear-encoded photosynthetic gene expression . In: Proc Natl Acad Sci USA 98 (4) pp. 2047-2052; PMID 11172073
- ↑ Pfannschmidt T., Schütze K., Brost M., Oelmüller R. (2001): A novel mechanism of nuclear photosynthesis gene regulation by redox signals from the chloroplast during photosystem stoichiometry adjustment . In: J Biol Chem 276 (39) pp. 36125-36130; PMID 11468291
- ↑ Schütze K., Steiner S., Pfannschmidt T. (2008): Photosynthetic redox regulation of the plastocyanin promoter in tobacco . In: Physiol Plant 133 (3) pp. 557-565; PMID 18419738
- ↑ Foyer CH and Noctor G. (2003): Redox sensing and signaling associated with reactive oxygen in chloroplasts, peroxisomes and mitochondria . In: Physiol Plant 119 (3) pp. 355-364
- ↑ Triantaphylidès C., Havaux M. (2009): singlet oxygen in plants: production, detoxification and signaling . In: Trends Plant Sci 14 (4) pp. 219-228; PMID 19303348
- ↑ Foyer CH, Noctor G., Buchanan BB, Dietz KJ, Pfannschmidt T. (2009): Redox Regulation in Photosynthetic Organisms: Signaling, Acclimation, and Practical Implications . In: Antioxidant Redox Signal 11 (4) pp. 861-905; PMID 19239350
- ↑ a b c Hope AB (2000): Electron transfers amongst cytochrome f, plastocyanin and photosystem I: kinetics and mechanisms . In: Biochim Biophys Acta 1456 (1) pp. 5-26; PMID 10611452
- ↑ Soriano GM, Ponamarev MV, Piskorowski RA, Cramer WA (1998): Identification of the basic residues of cytochrome f responsible for electrostatic docking interactions with plastocyanin in vitro: relevance to the electron transfer reaction in vivo . In: Biochemistry 37 (43) pp. 15120-15128; PMID 9790675
- ↑ a b Hervás M., Navarro JA, Molina-Heredia FP, De La Rosa MA (1998): The reaction mechanism of Photosystem I reduction by plastocyanin and cytochrome c 6 follows two different kinetic models in the cyanobacterium Pseudanabaena sp. PCC 6903 . In: Photosynth Res 57 (1) pp. 93-100
- ↑ Drepper F., M. Hippler, Nitschke W., W. Haehnel (1996): Binding dynamics and electron transfer between plastocyanin and photosystem I . In: Biochemistry 35 (4) pp. 1282-1295; PMID 8573585
- ↑ Sommer F., Drepper F., Hippler M. (2002): The Luminal Helix I of PsaB Is Essential for Recognition of Plastocyanin or Cytochrome c6 and Fast Electron Transfer to Photosystem I in Chlamydomonas reinhardtii . In: J Biol Chem 277 (8) pp. 6573-6581; PMID 11744732
- ↑ Sommer F., Drepper F., Haehnel W., Hippler M. (2004): The Hydrophobic Recognition Site Formed by Residues PsaA-Trp651 and PsaB-Trp627 of Photosystem I in Chlamydomonas reinhardtii Confers Distinct Selectivity for Binding of Plastocyanin and Cytochrome c6 . In: J Biol Chem 279 (19) pp. 20009-20017; PMID 14996834
- ^ A b Fromme P. (2003): Structure and function of photosystem I: interaction with its soluble electron carriers and external antenna systems . In: FEBS Lett 555 (1) pp. 40-44; PMID 14630316