Regitz diazo transfer
The Regitz diazo transfer is a name reaction in organic chemistry , in which a diazo donor is base-induced transferring a diazo group to a CH-acidic compound. Such a reaction was first published in 1910 by Otto Dimroth . However, it is named after Manfred Regitz , who in the 1960s took up the reaction, which had been neglected until then, and showed the general possibility of converting dicarbonyl compounds in the way described. The usual and more complex route of diazotization can thus be avoided, especially since it does not always lead to the desired product.
Mechanism of Regitz diazo transfer
Dimroth discovered the mechanism of Regitz diazo transfer by converting malonamide into sodium ethanolate using phenyl azide. The mechanism is explained more generally below using the example of a 1,3-dicarbonyl 1 : after adding a base such as. B. triethylamine ( 2 ) the dicarbonyl is first deprotonated in the α-position, the corresponding enolate 3 is formed . Due to its nucleophilic character, the enolate then attacks the diazo donor, here tosylazide ( 4 ).
In transition state I , the nitrogen of the azide bound to the sulfur of the tosylate then abstracts the second acidic proton on the carbon. The negative charge ( II ) created on the carbon then ensures that tosylamide ( 6 ) is split off as a leaving group; the diazo group remains on the carbon. So the product is a 2-diazo-1,3-dicarbonyl ( 5 ).
application
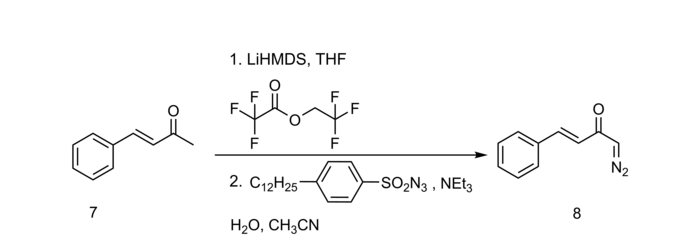
The Regitz diazo transfer offers advantages primarily through the possibility of using mild bases such as triethylamine , diethylamine or piperidine , as well as the convertibility of cyclic as well as acyclic 1,3-dicarbonyls. Simple cyclic as well as acyclic α-carbonyl compounds usually do not react directly with diazo donors and should therefore, for. B. be activated via a formylation analogous to the Claisen condensation. Higher yields, especially with base-sensitive compounds, are obtained by trifluoroacetylation:
The products resulting from the diazo transfer are important intermediates for a large number of reactions, for example for the Wolff rearrangement , more specifically also the Arndt-Eistert homologation , as well as for transition metal-catalyzed CH, NH and OH insertions or cyclopropanations.
Individual evidence
- ↑ Dimroth, O., et al. Intramolecular rearrangements. IV. Hydroxytriazoles and Diazocarboxylic Acid Amides. Ann. 1910 , 373 , 336-370, doi : 10.1002 / jlac.19103730306
- ^ Regitz, M. Reaction of active methylene compounds with azides. I. New synthesis of α-diazo-β-dicarbonyl compounds from benzenesulfonyl azides and β-diketones. Ann. 1964 , 676 , 101-109, doi : 10.1002 / jlac.19646760112
- ^ László Kürti , Barbara Czakó: Strategic Applications of Named Reactions in Organic Synthesis . Elsevier Science & Technology, Amsterdam, 2004 , p. 376, ISBN 0124297854 .
- ↑ Rick L. Danheiser, Raymond F. Miller, and Ronald G. Brisbois: Detrifluoroacetylative Diazo Group Transfer: (E) -1-Diazo-4-Phenyl-3-Butene-2-One In: Organic Syntheses . 73, 1996, p. 134, doi : 10.15227 / orgsyn.073.0134 ; Coll. Vol. 9, 1998, p. 197 ( PDF ).