Stephen Aldehyde Synthesis
The Stephen-aldehyde synthesis is a reaction of organic chemistry , it is in the synthesis of aldehydes from nitriles is. The reaction was first mentioned by Henry Stephen in 1925.
Overview reaction
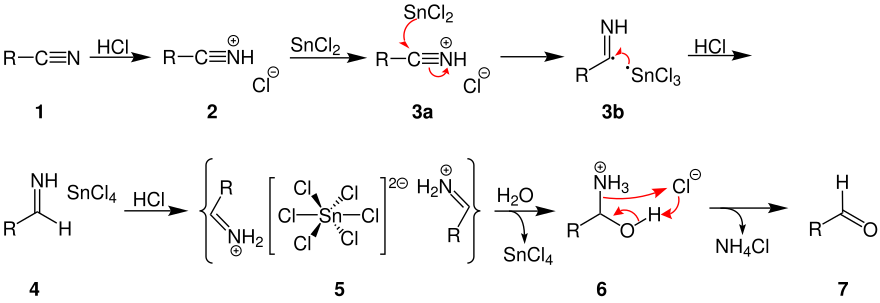
In this synthesis the nitriles are treated with stannous chloride to obtain crystalline aldimine stannous chloride which is then hydrolyzed .
Reaction mechanism
The postulated reaction sequence is explained below:
By adding hydrogen chloride , the nitrile ( 1 ) reacts in ether to form the corresponding salt ( 2 ). It is believed that this salt is reduced by one- electron transfer from the stannous chloride ( 3a and 3b ). The resulting salt ( 4 ) precipitates after some time as aldimine tin chloride ( 5 ). The hydrolysis of 5 produces a carboxamide ( 6 ) from which an aldehyde ( 7 ) is produced.
Substituents that increase the electron density favor the formation of the aldimine-tin chloride adduct. The formation of the amide chloride is facilitated by electron-withdrawing substituents. The success of the reaction used to be that the aldimine-tin chloride precipitated, washed with ether and then hydrolyzed. It has been found, however, that it is not necessary to remove the aldimine tin chloride from the solution. The preparation can be hydrolyzed directly in the solution.
This reaction is more productive when aromatic nitriles are used instead of aliphatic nitriles. However, the yield is also low with some aromatic nitriles (e.g. ethyl 2-formylbenzoate).
Individual evidence
- ↑ a b c d e f Zerong Wang: Comprehensive Organic Name Reactions and Reagents, 3 Volume Set . John Wiley & Sons, Hoboken, New Jersey 2009, ISBN 978-0-471-70450-8 , pp. 2659-2660.
- ↑ M. Rabinovitz: The Cyano Group (1970) . John Wiley & Sons, Chichester, UK 1970, ISBN 0-470-77124-0 , p. 308. doi: 10.1002 / 9780470771242.ch7