Strontium isotope analysis
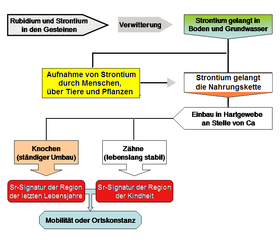
The strontium isotope analysis is used, among other things, to analyze the (pre) historical migration behavior of humans and animals. Depending on the geographical location, strontium is ingested with food in different isotope ratios and stored in bones and teeth. Archeology has been making increasing use of this new method for several years. The first projects originated in the USA and Great Britain and initially concerned North and Central America. Current applications deal, for example, with the migration behavior in the Upper Palaeolithic , the Neolithic or the Iron Age in Central Europe.
More recent applications use the effect of the geographically varying Sr isotopic composition also to determine the origin and authenticity of the food. Since, for example, certain regions are characterized by a characteristic Sr isotope signature, this signature is also reflected in various agricultural products, among other things. It should be noted, however, that the entry of fertilizer and precipitation can lead to an Sr isotope signature that deviates from the geological subsurface. This is also referred to as the “mobile Sr fraction”. Further applications of Sr isotope analysis can also be found in forensic medicine , for example .
The radioactive rubidium- strontium decay is suitable for dating rocks from the Precambrian .
Physical basics
Strontium has four stable, naturally occurring isotopes : 84 Sr (0.56%), 86 Sr (9.86%), 87 Sr (7.0%), and 88 Sr (82.58%). 87 Sr is formed in the β - decay of the rubidium isotope 87 Rb with a half-life of 4.81 · 10 10 a . Since the lifespan of 86 Rb, however, is only short, 86 Sr. is created during the formation of the rock. The isotope ratio of 87 Sr to 86 Sr gives an indication of the age of the rock. Depending on the region, the mean 87 Sr / 86 Sr ratio varies from 0.71 by up to 2%.
Storage in bones and teeth
Like calcium, strontium is an alkaline earth metal. Like calcium, it is therefore used in the body to build bones and teeth. Since the development of the teeth is completed in adolescence, one can deduce from this the region in which a person grew up, while the bones tell where they were in the last years of life. This makes it possible, for example, to analyze the migration movements of sedentary populations if the strontium isotopes from fossil finds are used for this purpose.
Benefits and Disadvantages
The prerequisite for the application is a. the mapping of rocks. A mapping of the strontium isotope ratios inevitably leads to ambiguities. Therefore, strontium isotope analysis alone is not sufficient. For the analysis of migration, therefore, additional evidence must be included, such as non-cultural artifacts in graves.
Another disruptive factor is the trade in food over long distances. This makes interpretation more difficult for cultures like ancient Rome, but it can also provide knowledge of food distribution.
Rubidium-Strontium dating
The half-life of 87 Rb is approximately 10 times longer than the estimated age of the earth. Rubidium-strontium dating is based on the decay of rubidium into strontium. With it, rocks from the Precambrian 4.5 billion years ago to the Cretaceous period about 50 million years ago can be classified. Other geochronological methods are preferable for younger rocks .
Thanks to the presence of the stable reference isotope 86 Sr, radiometric dating is based on the isochronous method . This makes it less sensitive to the estimation of the initial concentration and achieves an accuracy of up to 5%.
86 Sr - 87 Sr ratio in sediments and strontium isotope stratigraphy
Due to the decay of 87 Rb to 87 Sr, the proportion of this isotope in seawater in relation to 86 Sr increases steadily over time (due to the long residence time of the element in relation to the mixing, the content of the seawater is globally almost homogeneous at all times). This allows the ratio of these isotopes to be used to date marine sediments. Mainly biogenic minerals such as calcite and apatite as well as limestone and dolomite rocks are used, in which strontium is regularly stored as a small admixture in exchange for calcium. When dating, it should be noted that the ratio of the isotopes is only linear over relatively short periods of time. Viewed over geological epochs, the values fluctuate strongly cyclically with a period of a little more than 60 million years; therefore a calibration curve must be used as a basis for dating.
In addition to the absolute dating, the ratio of the strontium isotopes can be used for other interesting applications. Through fractional crystallization , the continental crust is enriched in strontium and rubidium compared to the earth's mantle . As a result, the proportion of 87 Sr in the weathering products of the continents and in the continental runoff is higher than in the mid-ocean ridges (fed from the mantle) . The ratio of 87 Sr to 86 Sr can therefore be used as an indirect indicator ( proxy ) for the entry of continental weathering products into the world's oceans, or to differentiate between marine and fluvial sediments. The rate of weathering and thus the isotope ratio is changed, for example, by the Ice Age ; this can be used for indirect dating of global ice ages, for example in the Precambrian . Numerous other applications are being tested. Sudden kinks in the curve of the isotope ratios in packages of marine sediments can be used to analyze the rate of deposition. Different conditions within a rock, for example the crystals and matrix of a sedimentary rock, clarify whether both are of the same age.
Individual evidence
- ↑ Thomas Prohaska, Maria Teschler-Nicola, Patrick Galler, Antonin Přichystal, Gerhard Stingeder, Monika Jelenc and Urs Klötzli: Non-destructive Determination of 87Sr / 86Sr Isotope Ratios in Early Upper Paleolithic Human Teeth from the Mladeč Caves - Preliminary Results. In: Early Modern Humans at the Moravian Gate. Springer, Vienna 2006. doi : 10.1007 / 978-3-211-49294-9 , ISBN 3-211-23588-4
- ↑ TD Price, J. Wahl, C. Knipper, E. Burger-Heinrich, G. Kurz, A. Bentley: The ceramic grave field of the 'Viesenhäuser Hof' near Stuttgart-Mühlhausen. New research results on migration behavior in the early Neolithic. In: Find reports from Baden-Württemberg. Stuttgart 27. 2003, 23-58. ISSN 0071-9897
- ^ TD Price, C. Knipper, G. Gruppe, V. Smrcka: Strontium Isotopes and Prehistoric Human Migration. The Bell Beaker Period in Central Europe. In: European Journal of Archeology. London 7.2004, 9-40. ISSN 1461-9571
- ↑ Karin Margarita Frei, Irene Skals, Margarita Gleba, Henriette Lyngstrøm: The Huldremose Iron Age textiles, Denmark - an attempt to define their provenance applying the strontium isotope system. In: Journal of Archaeological Science. Oxford 36.2009,9, pp. 1965-1971. doi : 10.1016 / j.jas.2009.05.007 , ISSN 0305-4403
- ^ Decay Radiation Results . In: Chart of Nuclides database . National Nuclear Data Center. Retrieved January 24, 2012.
- ↑ isotopic distribution for UK.
- ↑ JM McArthur, RJ Howarth; GA Shields: Strontium Isotope Stratigraphy. Chapter 7 in Felix Gradstein, James Ogg, Mark Schmitz, Gabi Ogg (Editors): The Geologic Time Scale 2012. Elsevier. ISBN 9780444594259 . doi : 10.1016 / B978-0-444-59425-9.00007-X
- ^ A b Jan Veizer (1989): Strontium Isotopes in Seawater Through Time. Annual Review of Earth and Planetary Sciences, Vol. 17: 141-167.
- ↑ Adrian L. Melott, Richard K. Bambach, Kenni D. Petersen, John M. McArthur (2012): A ~ 60 Myr periodicity is common to marine 87Sr / 86Sr, fossil biodiversity, and large-scale sedimentation: what does the periodicity reflect? Journal of Geology 120: 217-226.
- ^ GA Shields-Zhou, AC Hill, BA Macgabhann: The Cryogenian Period. Chapter 17 in Felix Gradstein, James Ogg, Mark Schmitz, Gabi Ogg (Editors): The Geologic Time Scale 2012. Elsevier. ISBN 9780444594259 . doi : 10.1016 / B978-0-444-59425-9.00017-2
Web links
literature
- O. Hahn , F. Strassmann and E. Walling: Production of weighable quantities of the strontium isotope 87 as a conversion product of rubidium from a Canadian mica. In: Naturwissenschaften, 25, 189, 1937.
- O. Hahn and E. Walling: About the possibility of geological determination of the age of rubidium-containing minerals and rocks. In: Zs. F. Anorg. Chemie, 236, 78-82, 1938.
- O. Hahn: Geological age determination using the strontium method. In: Chemiker-Zeitung, 67, 55, 1943.