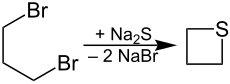Thietane
| Oxetanes |
|---|
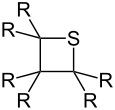 Thietane (general formula) |
 Thietan |
Thietanes are heterocyclic organic chemical substances that contain a four-membered ring consisting of one sulfur atom and three carbon atoms. The unsubstituted parent of this group of substances is thietane with the empirical formula C 3 H 6 S.
Manufacturing
1,3-Dibromopropane reacts with sodium sulfide (Na 2 S) with cyclization to the corresponding thietane:
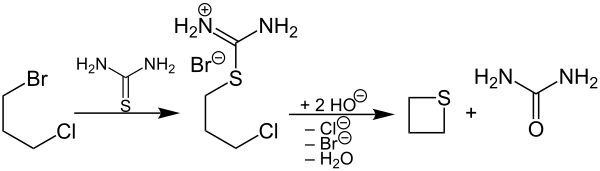
A salt can be produced from 1-bromo-3-chloropropane by reaction with thiourea , the basic hydrolysis of which gives thietane and urea :
This synthesis method usually gives better yields than the former, but is not very atom-efficient , since several waste materials with a comparatively high molar mass are formed in at least a stoichiometric amount.
Reactivity
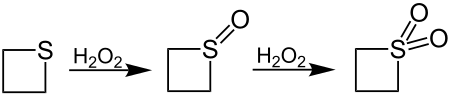
With hydrogen peroxide (H 2 O 2 ) thietane is oxidized to the sulfone via the sulfoxide stage :
Web links
Individual evidence
- ↑ a b c d Theophil Eicher , Siegfried Hauptmann, Andreas Speicher: The Chemistry of Heterocycles , Wiley-VCH, 2012, ISBN 978-3-527-32747-8 , pp. 49-50.