Van Leusen reaction
The Van Leusen reaction is a name reaction in organic chemistry for the synthesis of nitriles . To do this, a ketone is reacted with tosylmethyl isocyanide (TosMIC). This reaction was first described in 1977 and is named after its discoverer, the Dutch chemist Albert van Leusen (* 1933).
Overview reaction
The reaction takes place between a ketone and tosylmethyl isocyanide. The structural formula of tosylmethyl isocyanide, which is abbreviated to TosMIC in the following, can be seen in the adjacent figure. The reaction of acetone to isobutyronitrile is shown here as an example :
Under certain conditions, the Van Leusen reaction can also be carried out with aldehydes.
Reaction mechanism
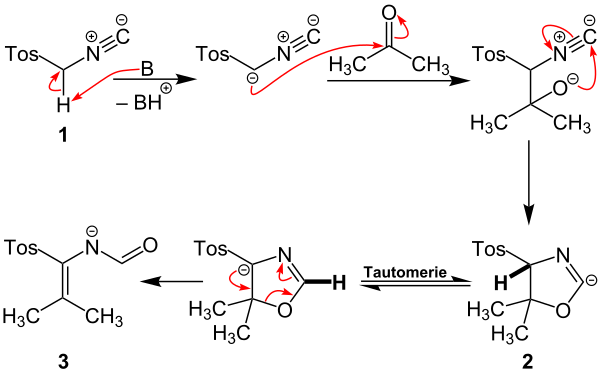
A possible reaction mechanism for the Van Leusen reaction will now be explained using the example of acetone. For the sake of simplicity, the tosyl group of the TosMIC molecule, which is marked green in the structural formula above, is abbreviated to "Tos".
The reaction starts with the deprotonation of TosMIC 1 to form a carbanion that is stabilized by the neighboring sulfonyl and isonitrile groups. The carbanion nucleophilically attacks the carbonyl carbon atom of acetone. A ring closure takes place via electron migration, so that intermediate stage 2 is created. This reacts via tautomerism and ring opening to form reactive intermediate 3 .
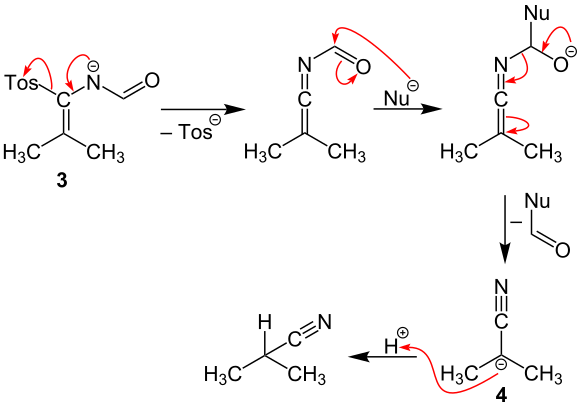
In the next step, the tosyl radical is split off from the open-chain intermediate 3 . A nucleophilic particle (for example an aloholate) is then attacked on the carbonyl carbon atom . A subsequent elimination reaction produces intermediate 4 , which reacts by protonation to form the end product, isobutyronitrile.
Individual evidence
- ↑ a b Otto Oldenziel, Daan van Leusen, Albert van Leusen: Chemistry of sulfonylmethyl isocyanides. 13. A general one-step synthesis of nitriles from ketones using tosylmethyl isocyanide. Introduction of a one-carbon unit . In: American Chemical Society (ed.): J. Org. Chem. . 42, No. 19, 1977, pp. 3114-3118. doi : 10.1021 / jo00439a002 .
- ↑ a b Daan van Leusen, Albert van Leusen: Synthetic Uses of tosylmethyl isocyanides (TosMIC) . In: Organic Reactions . 57, No. 3, 2004, pp. 417-666. doi : 10.1002 / 0471264180.or057.03 .